57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR1050070-UR10: Controlling Morphology in Bulk Heterojunction Solar Cells via Chemical Design and Surface Patterning Methods
Lee Y. Park, PhD, Williams College
We are continuing our work on both fluorinated copolymers based on polyphenylenevinylene materials, and have also begun work on small molecule analogs for possible use in bulk heterojunction solar cells.

 For the polymeric materials, we studied
the absorption profiles of fluorinated and non-fluorinated analogs on
fluorinated and non-fluorinated surfaces.
The fluorinated polymers consistently give broadened absorption profiles
relative to their non-fluorinated analogs, and the absorption envelope is
further broadened when films are cast onto fluorinated surface, as illustrated
in the Figure above.
For the polymeric materials, we studied
the absorption profiles of fluorinated and non-fluorinated analogs on
fluorinated and non-fluorinated surfaces.
The fluorinated polymers consistently give broadened absorption profiles
relative to their non-fluorinated analogs, and the absorption envelope is
further broadened when films are cast onto fluorinated surface, as illustrated
in the Figure above.
We also see evidence (see Figure to the right) for more efficient fluorescence quenching of the fluorinated polymers by PCBM (the fullerene derivative typically used as an acceptor material in bulk heterojunction solar cells) compared with non-fluorinated analogs.
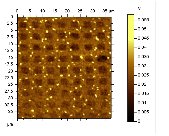
Finally, we worked on fluorinating PEDOT-PSS via microcontact printing in anticipation of preparing devices in which we can cast our fluorinated materials onto an appropriate fluorinated surface in order to study actual photovoltaic efficiencies. Shown to the right is an AFM lateral force image of a PEDOT-PSS surface that has been microcontact printed with a fluorinated ink in a pattern consisting of a square array of circular holes.
The results of this work to date were presented at the National Meeting of the American Chemical Society in San Diego in March 2012 (undergraduate coauthors indicated by asterisks): "Partially fluorinated sidechains in influencing polymer film morphology, absorption and fluorescence behavior" Daniel A. Gross,* Cameron R. Rogers,* Katrina A. Tulla,* Erica L. Wu,* Lee Y. Park.
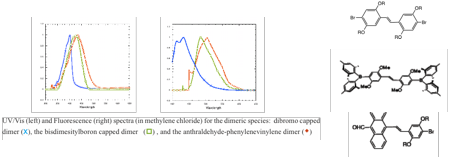
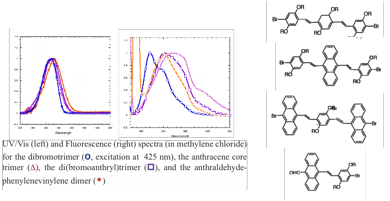
In addition, we began to investigate small molecule analogs of polyphenylene vinylenes as well. We developed a modular synthetic route to a series of oligophenylenevinylenes in which core and endgroup substituents can be varied systematically in order to study the impact of the structural variations on electronic and optical properties. We prepared our first examples of dimeric and trimeric species involving phenyl and anthryl core and terminal units, as well as bromine-, dimesitylboron-, and aldehyde- based capping substituents. We studied the UV/Vis and fluorescence properties of the dimeric and trimeric families that we've prepared thus far, as illustrated in the two Figures shown below. We are currently working to develop a more complete library of related compounds in order to systematically investigate the effect of electron withdrawing groups and various aromatic cores. This work was presented at the National Meeting of the American Chemical Society in San Diego as well (undergraduate coauthors indicated by asterisks): "Optical and electronic properties of discrete phenylenevinylene-based oligomers" Grace C. Babula,* Chelsea D. Boydstun,* Peter L. Clement,* Christopher J. Corbett,* Alejandro R. Gimenez,* Mindy C. Lee,* Lee Y. Park.