57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR349503-UR3: In Situ Thermodynamic Characterization of Supramolecular Assembly Processes that Lead to Discrete Nanosize Structures
Douglas A. Vander Griend, Calvin College
In our quest to study chemical systems with high degrees of organizational, dynamic, and compositional complexity, we have several new major scientific results to report this year. For details of previous results, please see the annual report submitted in September of 2011.
After establishing the usefulness of spectrophotometric titrations for investigating complicated solution dynamics, we took on an even bigger challenge than last year: elucidating the thermodynamic assembly of twenty molecular pieces into a supramolecular cube (Figure 1). The original chemistry was designed by Dr. Michael D. Ward of Sheffield University, but neither he nor anyone else had studied the solution-phase assembly of this twenty-piece nanocontainer. This represents the most complicated self-assembly process characterization that we are anyone else has undertaken as far as we know.
Ni2+
+
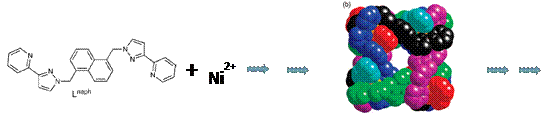
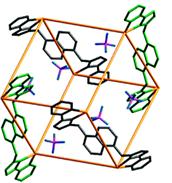
Figure 1. Twelve Lnaph ligands (two bidentate pyrazolyl–pyridine termini separated by a 1,5–naphthalene) and 8 nickel(II) cations form a cubic nanocage in acetonitrile or DMF solution.
Figure 2. The supramolecular nanocage in the solid state has been previously characterized by X-ray diffraction.
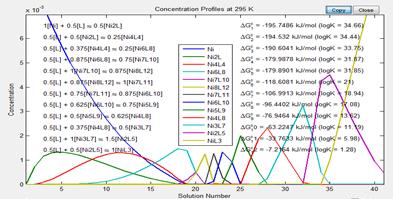
By titrating ligand into an acetonitrile solution of Ni(BF4)2, the color was observed to shift only subtlety. However, spectroscopic measurements of the absorbance from 450 to 850 nm could be modeled to reveal the presence of 12(!) different supramolecular structures. Figure 3 shows the how the data deconvoluted to molar absorptivity spectra and concentration profiles for thirteen distinct species, including [Ni(MeCN)6]2+.
Figure 3. The spectral data from approximately 50 different solutions of varying ratios of ligand and nickel cation could be deconvoluted into molar absorptivity curves (a) and concentration profiles (b) for thriteen different absorbing species.
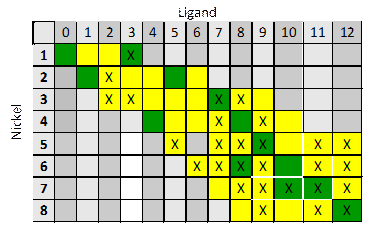
These data represent 'snapshots' of the assembly of the cubic nanocontainer in which numerous distinct partial structures could be identified. The best model found means that adding any more species led to molar absorptivity curves that were non-sensical, and no other combination of species led to a better fit with the data. Figure 4 depicts the different make-up of the various supramolecular complexes found in solution according to our best models. The course of the titration starts with NiL3 and moves right and down until it reaches the cube, which is the bottom right box. Further titration leads to species with more and more nickel until the last species, Ni2L forms. Additional nickel(II) added to the solution beyond this point is uncoordinated.
Figure 4. The number across the top is the number of ligand molecules in the structure. The number down the right is the number of nickel(II) cations in the structure. Colored boxes, both green and yellow, correspond to species that could have produced the mass spectrometry data. An 'X' indicates that the species was unambiguously evidenced with mass spec. Green boxes are those that were also part of the best model of spectrophotometric data.
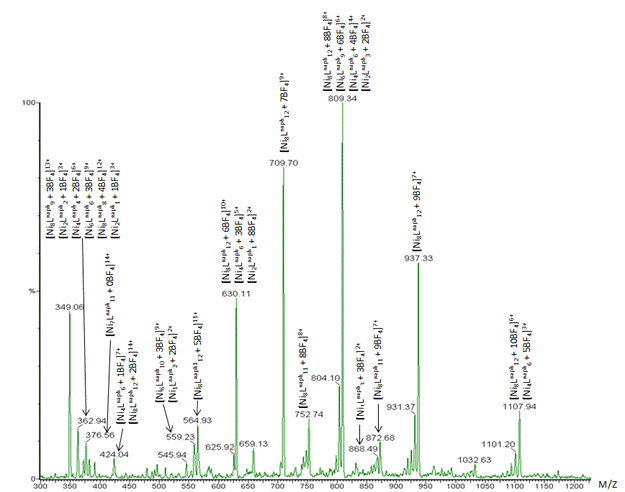
Obviously there is still quite a bit of uncertainty when it comes to definitively identifying all the partial assemblages, and that is where we have turned to mass spectrometry. Figure 5 displays an example of data that we took at Michigan State University under the tutelage of Prof. Daniel Jones. By keeping the cone voltage on the instrument sufficiently low, we could see evidence for supramolecular assemblages that stayed together. Unfortunately, we did not have sufficient resolution to simultaneously determine the absolute charge. With the new instrument that MSU is installing this fall, however, we will be able to do both. The combination of spectrophotometric mass spectrometry data should help us to finally and fully characterize this tremendously complicated solution system.
Figure 5. Mass spectrometry data for a 1:1 solution of Ni(II) and Ligand. Supramolecular complexes are found intact, but resolution is not sufficient to determine the absolute charge.
Besides studying the assembly of the supramolecular cube, we also investigated the solution dynamics of the dye methylene blue in order to sort out the limitations of modeling systems. We have discovered and quantified the extent to which signal noise obscures a true model from other good ones by testing copious amounts of simulated data.
The impact of the research into equilibrium self-assembly over the last 12 months has been substantial. Three undergraduates continued in research for a second summer while two new students joined my lab for the first time this past summer. Four of them joined me in Philadelphia this August for the national ACS meeting and all testified that the experience definitely excited them for more research. Four of the five continue to conduct research now that the school year has begun (the fifth is studying abroad this semester). Two of them should submit paper based on their work this fall. One student in particular has done quite a bit to make our modeling program accessible to the world and we hope within the next year to launch it via the internet.
As for my career, I continue to work with collaborator Dario Pasini of the University of Pavia, Italy; and Michael Ward of Sheffield University, England; as well as interact to various degrees with several other researchers who have asked for help in characterizing their particular ensemble of compounds. I continue to champion the power of these techniques as well as the wide ranging applications as I give seminars around the country.
Thank you for supporting this research and making the impact possible.