57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI351217-UNI3: Functionalized, Binuclear N-Heterocyclic Carbene Ligands Bearing Phosphine Donors: Synthesis of Metal Complexes and Applications to Homogeneous Catalysis
Gregory J. Domski, PhD, Augustana College
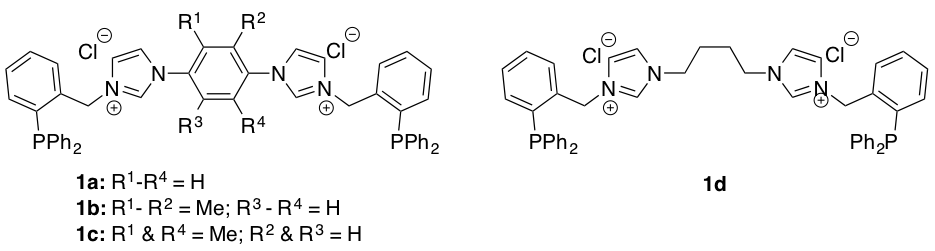
During the 2011 – 2012 academic year we pursued the synthesis of the benzyl-linked phosphine-functionalized bisimidazolium salts described in the original proposal (Figure 1). We successfully prepared and isolated two of these salts (1a and 1b), however the yields were quite low (ca. 36 % and 11 % respectively) and inconsistent. The major product obtained from our syntheses in most cases was the monoimidazolium salt. This observation led us to hypothesize that the second substitution reaction was not proceeding as expected. We explored several potential explanations including: low solubility of the monoimidazolium salt, reactant-favored equilibrium, and high activation energy for the second substitution reaction. The results of our experiments are summarized in Table 1.
Figure 1. Initially targeted bisimidazolium salts
Table 1. Results of bisimidazolium salt synthetic trials
Desired bisimidazolium
| Benzyl chloride (eq)
| Bisimidazole (eq)
| Solvent
| Temperature (°C)
| Duration (hours)
| Mono : Bisa | Bisimidazolium isolated
|
1a | 2 | 1 | DMF | 80 | 16 | n.d.b | 36 % |
1b | 2 | 1 | DMF | 90 | 42 | 70:30 | 11 % |
1c | 2 | 1 | DMF | 90 | 112 | 60:40 | none |
1c | 2 | 1 | iPrOH | 70 | 93 | n.a.c | none |
1c
| 2 | 1 | 8:1 THF/EtOH | 80 | 96 | 70:30 | none |
1c | 2 | 1 | Toluene | 110 | 48 | n.a. | none |
1c
| 3 | 1 | DMF | 90 | 48 | 70:30 | none |
1c
| 3 | 1 | EtOH | 78 | 72 | 60:40 | none |
1c | 2.5 | 1 | EtOH | 78 | 96 | 60:40 | none |
1d
| 2 | 1 | DMF | 80 | 96 | 40:60 | none |
1c | 2 | 1 | neat, vacuum | 150 | 96 | n.a. | none |
aDetermined via 1H NMR spectroscopy, bn.d. = not determined, cn.a. = not applicable since reaction showed neither mono- or bisimidazolium via 1H NMR spectroscopy.
In order to probe the solubility issue several different solvents were employed including: toluene, ethanol (EtOH), isopropanol (iPrOH), tetrahydrofuran (THF), and N,N-dimethylformamide (DMF). One mixed solvent system was also investigated: 8:1 THF/EtOH. The only solvent from which we were able to successfully isolate the bisimidazolium salt was DMF. Ethanol also gave some promising results, however ethanol competed with the bisimidazole and monoimidazolium as a nucleophile further complicating purification efforts. In many cases significant amounts of bisimidazolium were observed via 1H NMR spectroscopy, but isolation proved difficult. Purification was achieved serendipitously in some cases (i.e. the bisimidazolium precipitated from the reaction mixture), and in other cases trituration of the crude reaction mixture in diethyl ether (Et2O) furnished the bisimidazolium. Column chromatography on silica using 75:25 CH2Cl2/MeOH allowed us to isolate the monoimidazolium salts, however, we were unable to isolate the bisimidazolium salts even after eluting with pure methanol.
We tried to force the equilibrium position of the second substitution towards products by using an excess of phosphine-functionalized benzyl chloride in several experiments. Instead of improving yields, using an excess of the benzyl chloride led to the formation of an as of yet unidentified byproduct thereby further complicating the purification process.
In an attempt to overcome the presumed high activation energy barrier we carried out the substitution reaction at high temperature (150 °C) in the absence of solvent and under reduced pressure. The 1H NMR spectrum of the crude product from this experiment showed only unreacted bisimidazole and the aforementioned unidentified byproduct.
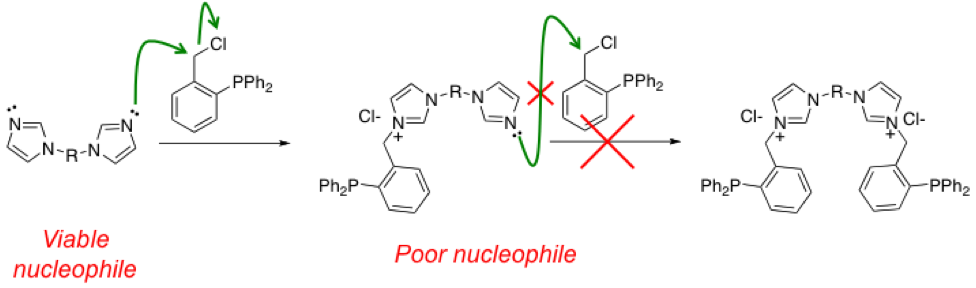
Our current hypothetical explanation for the poor yields of the targeted bisimidazolium salts and the apparent preference for the monoimidazolium invokes the electron withdrawing nature of the imidazolium moiety. After the first substitution occurs, the positively charged imidazolium group may draw electron density away from the imidazole thereby decreasing its electrophilicity and weakening its nucleophilic potential (Scheme 1). If this is the case, there is little that can be done to overcome this synthetic problem.
Scheme 1.
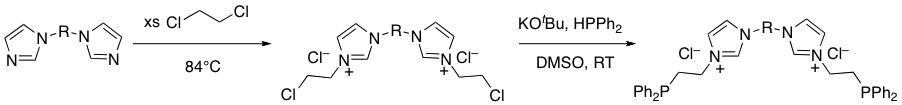
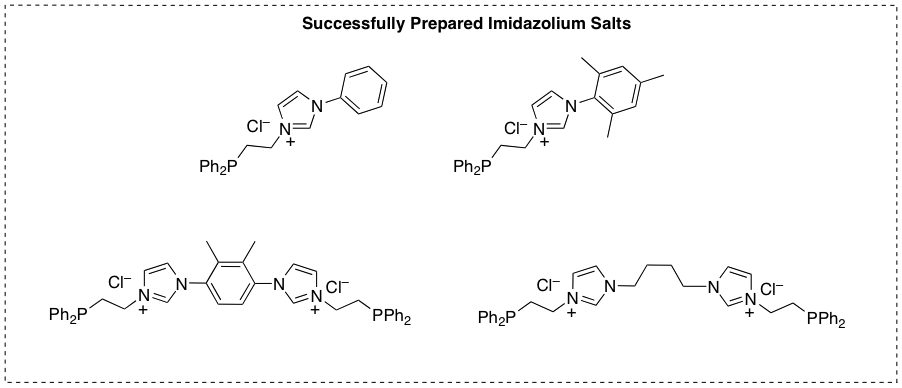
In light of the difficulties inherent in our initial synthetic approach, we decided to pursue a slightly different class of phosphine-functionalized bisimidazolium salts through a synthetic pathway that reversed the identities of the nucleophile and electrophile (Scheme 2) during summer 2012. This pathway has been quite successful; we have used it to prepare several phosphine-functionalized mono- and bisimidazolium salts (Figure 2).
Scheme 2.
Figure 2. Successfully prepared ethane-linked phosphine-functionalized mono- and bisimidazolium salts
Future work will focus on metalation of ethane-linked phosphine-functionalized N-heterocyclic carbenes first with silver (I) and then transmetalation onto other metals with ruthenium(II) being the primary target of metalation. After successful metalation we will evaluate the potential of the mono- and bimetallic ruthenium(II) complexes to catalyze the transfer hydrogenation of ketones.