57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR749407-UR7: Synthesis of Nanoparticle-Cored Dendrimers with Single Molecular Weight Using Dendritic Functionalization of Monodisperse Gold Nanoparticles
Young Shon, California State University (Long Beach)
Progress Report 2011-2012
The primary goal of this research is to develop a synthetic pathway to generating monodispersed Au25 nanoparticle-cored dendrimer (AuNCD). There are several approaches recently developed in synthesizing AuNCD, however, the synthesis of AuNCD while maintaining a consistent size has always been problematic for researchers over the years. The availability of highly monodisperse NCDs will allow us to further elucidate the relationships between primary structural elements in these nanostructures and their chemical, physical and biological properties.
To address the problems encountered in the previous periods, we took a more direct method in generating monodispersed AuNCD. The first step was the synthesis of water soluble Au25Ligand18, with glutathione as the ligand of choice, eliminating needs of ligand exchange. The synthesis of glutathione-protected Au25 nanoparticles was followed by the process of dendron coupling using EDC (1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride) in the presence of NHS (N-hydroxysuccinimide) and MES (2-(N-morpholino)ethanesulfonic acid, pH= 4.7-6) buffer (Scheme 1). The use of these reagents and reaction condition turned out to be critical for a successful coupling reaction and eliminated the formation of particle aggregates.
| |
| |
|
|

Scheme 1. Synthesis of PFd-G3-ArNH2-OH-capped Au25 NCD.
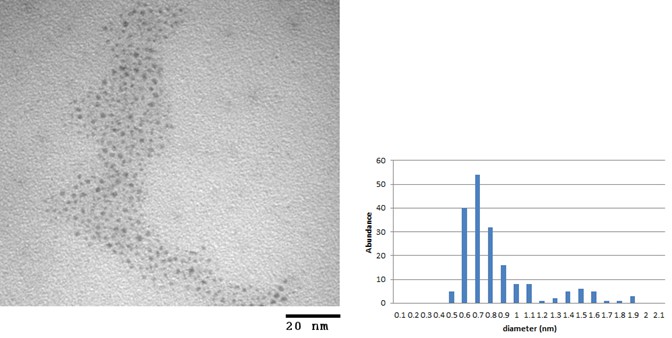
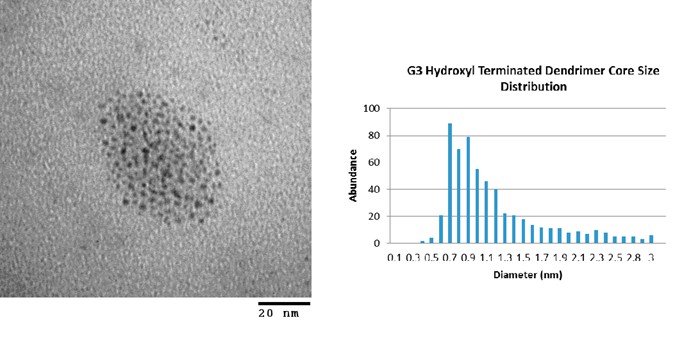
The two dendrons used for the coupling reactions were G1-aminotriester and water-soluble PFd-G3-ArNH2-OH. A combination of UV-visible spectroscopy and transmission electron microscopy (TEM) was used to monitor the changes, if any, to the Au25 clusters while infrared (IR) spectroscopy and thermogravimetric analysis (TGA) were used to identify changes to the organic coverage. UV-vis analysis clearly showed distinct peaks corresponding to molecule-like electronic levels of Au25 nanoparticles (absorption bands at 680 nm, 440 nm, and 400 nm) even after dendron coupling reactions. Since the unique spectroscopic UV-visible absorption spectra of the Au25 core allows us to determine any changes to the size of the gold core as we proceed through the synthesis, the result clearly suggested a preservation of Au25 core throughout the process. TEM and TGA analysis revealed that the prior to the coupling of the dendrons, the Au25 nanoparticle contained Au clusters with the average diameter of 0.99 nm with the standard deviation (STD) of ± 0.37 nm with organic content of 39.2%. The resulting G1-aminotriester-capped and PFd-G3-ArNH2-OH-capped Au25 NCD contained Au clusters with the average diameter of 1.01 ± 0.31 nm and 1.08 ± 0.32 nm (Figure 1) with an organic layer of approximately 45.02% and 43.3%, respectively. TGA analysis confirmed an increase in 4-6 % of organic fractions when compared to the TGA results of Au25SG18 precursor. TGA data were correlated to about 2-3 dendron attachments to each Au nanoparticle. IR spectra of obtained AuNCDs showed a peak at 1646 cm-1 suggesting the presence of amide bonds. The spectra also showed a large O-H and N-H stretching absorption bands within the region between 3500-2500 cm-1. In addition, IR spectra showed evidence of ester carbonyl stretching at 1724 cm-1 and a disappearance of a carboxylate carbonyl stretching in the 1630-1522 cm-1 region previously observed for the Au25 NP precursor. The disappearance of the carboxylate carbonyl stretching could prove the occurrence of dendron attachment and the presence of both glutathione and dendrons in the organic layer.
(a) (b)
Figure 1. TEM images (left) and core size histograms (right) of (a) Au25SG18 G1 aminotriester NCD and (b) Au25SG18 PFd-G3-ArNH-OH NCD.
One manuscript is currently in preparation to disseminate this research. An extended proposal based on the obtained results has now been funded by NIH (NIGMS-SC3 program) for four years (2011-2015). The primary goal of this NIH supported research is to prepare stable and biocompatible nanoparticle (NP) platform for multifunctional diagnostics and therapeutic agents (hyperthermia treatment guide).
An alternate project on the investigation of alkanethiolate-capped Pd nanoparticles that can act as selective catalysts has been continuously productive. The research during this period was focused on understanding the mechanism and the regioselectivity of Pd nanoparticles in different environments (ref. 2). Pd nanoparticles were found to convert allyl alcohol selectively to either propanal or 1-propanol depending on the type of solvent used for the catalytic reactions. The reaction pathway was most likely determined by steric hindrance, which was the result of the interaction between substrate and alkylthiolate ligands on Pd nanoparticles. In general, nonpolar or weakly polar solvents such as benzene and chloroform, respectively, promote the isomerization of allyl alcohol to propanal via the formation of the branched Pd-alkyl intermediate. On the other hand, polar protic solvents such as methanol and water foster the hydrogenation of allyl alcohol to 1-propanol involving the steric induced formation of linear Pd-alkyl intermediate.
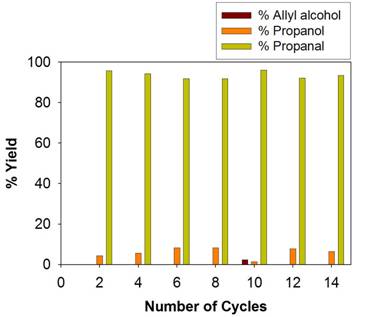
The relationship between the catalytic property and the surface ligand density/core size of thiolate ligand-capped Pd nanoparticles (PdNPs) was also investigated (ref. 1). The decrease in the molar equivalent of sodium S-dodecylthiosulfate (Bunte salts) resulted in the formation of nanoparticles with lower surface ligand density and larger particle core size. A decrease in the molar equivalent of tetra-n-octylammonium bromide or an increase in reaction temperature generated nanoparticles with higher surface ligand density and smaller particle core size. As the molar equivalent of NaBH4 decreased, the particle core size increased. The catalysis studies on various PdNPs with different surface ligand density and average core size showed a strong correlation between the PdNP composition and the turnover frequency (TOF) of the isomerization of allyl alcohol. Optimized "Good" PdNPs with lower surface ligand coverage and larger core size catalyzed the isomerization of various allyl alcohols to carbonyl analogues with high activity and selectivity. Lastly, the high stability of soluble nanocatalysts is demonstrated by recycling dodecanethiolate-capped Pd nanoparticles over ten times for the isomerization reaction of allyl alcohol (Figure 2).
Figure 2. The recycling profiles of the catalytic isomerization of allyl alcohol using dodecanethiolate-capped Pd nanoparticles.
This project produced two peer-reviewed publications during this 2011-2012 period (a total of four publications during three-year PRF grant period). We are currently in preparation of two more research papers related to nanoparticle catalysis project.