57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR450664-UR4: Synthesis of Diarylnorbornadiene Derivatives and their Potential Use in Solar to Thermal Energy Conversion
Felix E. Goodson, West Chester University of Pennsylvania
Karyn Usher, PhD, West Chester University of PA
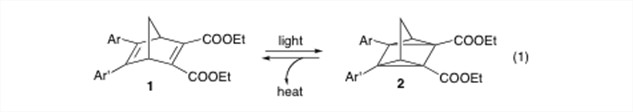
Our research has focused on the synthesis of a series of diarylnorbornadiene compounds (1). These compounds are of interest because the norbornadiene can undergo a [2+2] cycloaddition to form the highly strained quadricyclane (2) (eq. 1). Since this strain energy could later be released in the form of heat, these compounds have been envisioned for applications in the area of solar to thermal energy conversion.1
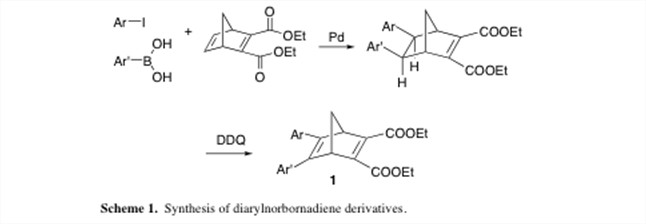
The traditional means of synthesizing these compounds relied on a multistep route.2 However, we have developed a two-step procedure from readily available starting materials (Scheme 1). In this manner we have synthesized a library of diarylnorbornadiene derivatives in which groups on one of the aromatic rings have been independently varied.
The first step of this synthesis is based upon a reaction previously developed in our laboratory.3 While the second step appears simple, the purification of the products has proved to be nontrivial. However, we have been successful in utilizing the reversible photochemistry of these compounds to purify them via preparative reverse-phase HPLC. While the norbornadienes tend to exhibit longer retention times, the quadricyclanes typically elute much more quickly. As a result, we can inject a crude sample of the quadricyclane, collect the earlier eluting peaks (and thereby eliminate the impurities with later retention times), promote the thermal reversion to the norbornadiene, reinject the sample, and then collect the later peak. To date, we have purified thirteen diarylnorbornadiene compounds using this, as well as more standard methodologies.
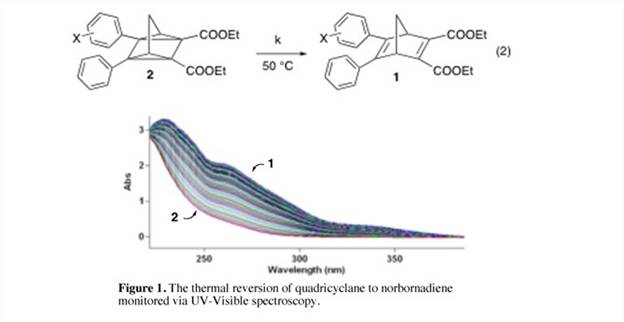
A second focus of our research has been to study how substituents on one or more of the benzene rings affect the thermal stability of the quadricyclanes. By monitoring the kinetics of the reversion (eq. 2, Figure 1) via UV-Visible spectroscopy, we determined that electron-withdrawing groups in the meta position slowed down the reversion, and thus had a stabilizing effect on the quadricyclanes. A standard Hammett analysis of the data resulted in a slightly negative slope, but a poor linear fit. The 4-nitro and 4-cyano substituents were significant outliers in the plot, accelerating the reaction while other electron-withdrawing substituents slowed it down. A similar analysis utilizing sigma constants from hyperfine splitting of substituted benzylic radicals4 resulted in a much better fit, suggesting that the quadricyclane to norbornadiene reversion follows a free-radical pathway, in agreement with earlier suggestions.5
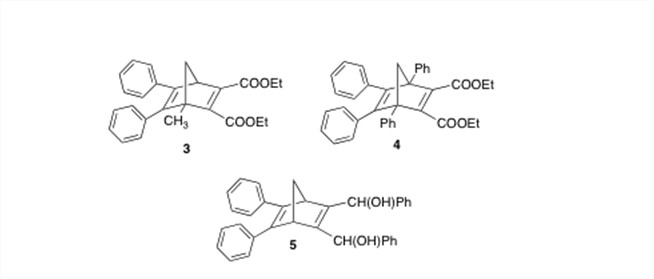
A third direction of our research has been to develop the "next generation" of the norbornadienes that we wish to study. Unfortunately, none of the derivatives in our first library form a stable quadricyclane, unlike some of the compounds originally presented by Hirao.2 The key difference is the presence of methyl groups at the bridgehead positions. Could these methyl groups be having such a profound effect on the stability of the quadricyclanes? Also, considering the apparent free radical mechanism of the quadricyclane reversion, would it be better to replace the ester groups with substituents that would not stabilize radical intermediates through resonance? To investigate this possibility, we have synthesized compounds 3-5, and we are presently studying their photochemical properties.
To date, summer research opportunities for six undergraduate students have been fully or partially supported by this grant. Of these six, one has graduated and is currently pursuing his Ph.D. Three of the six presented their work on this project at the recent American Chemical Society national meeting in Philadelphia. All six of these students have learned skills in organic synthesis, liquid chromatography, and the analysis of kinetics data. Of the five students involved who will be graduating this spring, four are considering graduate school in chemistry.
In terms of the impact of this research on the careers of the principal investigators, the work in this project would not have been possible without the collaboration with Dr. Usher, whose expertise has enabled all of us involved to learn about the advances in liquid chromatography. Involvement in this research has similarly had an impact on Dr. Usher's career by introducing her to organic techniques and methodologies that she had not previously experienced.
References.
1. Dubonosov, A. D.; Bren, V. A.; Chernoivanov, V. A., Russian Chem. Rev. 2002, 71, 917-927.
2. Hirao, K.; Ando, A.; Hamada, T.; Yonemitsu, O., J. Chem. Soc., Chem. Commun. 1984, 300-302.
3. Shaulis, K. M.; Hoskin, B. L.; Townsend, J. R.; Goodson, F. E.; Incarvito, C. D.; Rheingold, A. L., J. Org. Chem. 2002, 67, 5860-5863.
4. Dust, J. M.; Arnold, D. R., J. Am. Chem. Soc. 1983, 105, 1221-1227.
5. Kabakoff, D. S.; Bünzli, J.-C. G.; Oth, J. F. M.; Hammond, W. B.; Berson, J. A., J. Am. Chem. Soc. 1975, 97, 1510-1512.