57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI550427-DNI5: Tuning Dihydrogen Adsorption at Metal Centers of Surface-supported Supramolecular Networks
Steven L. Tait, PhD, Indiana University
This project utilizes coordinatively-unsaturated metal centers in metal—organic frameworks at surfaces to study the tuning of these metal sites for di-hydrogen adsorption. The delicate challenge of binding the intact di-hydrogen molecule requires stable adsorption at the metal site through donation of σ electrons from H2 to unoccupied d orbitals of the metal, while avoiding excessive back donation to the H2 anti-bonding orbital. Here, we study a metal-organic complex stabilized at a solid surface to achieve these goals. The objective of the work is to develop protocols for metal-organic complexes at surfaces and evaluate their efficacy in providing a route to tunable surface functionalization. The study brings together the stability of surface supported nanostructures with the chemical tunability of metal-organic complexes.
In this project period, we have developed protocols for self-assembled nanostructures of 2,5-pyrazine dicarboxylic acid (PDA) on the Cu(100) surface, as well as its complexation with Cr on the same surface. This system provides a prototype for the adsorption of dihydrogen at a solid surface, a key step in important industrial processes.
Organic and metal components are vapor deposited to a surface. The experiments are conducted under ultra-high vacuum conditions (< 1 x 10-9 Torr) in order to maintain very well-controlled conditions and clean surfaces. We can control the surface coverage of each component in the system by the shutter open time of the evaporators. Studies in vacuum also allow the use of electron spectroscopy methods. For example, we have identified the adsorption of PDA using X-ray photoelectron spectroscopy. We also have capability to characterize local structure of the surface by high-resolution scanning tunneling microscopy (STM) with atomic resolution.
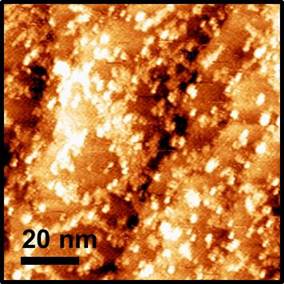
Figure 1 shows recent data on the self-assembly of PDA at the Cu(100) surface, imaged by STM. The local structural characterization afforded by STM allows us to focus our study on the fundamental interaction at work in the self-assembly of the organic nanostructures and metal-organic complexes at the surface.
Figure 1. High-resolution scanning tunneling microscopy image of 2,5-pyrazine dicarboxylic acid (PDA) on the Cu(100) surface. Single PDA molecules are imaged as bright dots. The molecules have assembled into one dimensional chains which are oriented along the low index directions of the crystalline copper surface. Image size is 20 nm x 20 nm.
The structure of the resulting self-assembled nanostructure in Figure 1 is striking due to the one-dimensional assembly of the molecules at the surface. The PDA molecules arrange themselves in chains along the low-index [0 1 1] direction of the copper surface and the symmetrically equivalent [0 -1 1], which are separated by 90°. The molecules acquire one of two surface chiralities as they adsorb to the surface, so that they have either a left or right orientation, as illustrated in Figure 2. The chain assemblies of the molecule consist of a single molecule adsorption chirality.
Figure 2. Molecular models of PDA chains on Cu(100). The molecules adsorb in one of two surface chiralities. One dimensional chains of PDA on the surface are enantiopure.
From the model shown in Figure 2, some insight can be provided as to the one dimensional nature of the supramolecular interaction. Deprotonated molecules likely interact by weak electrostatic interactions or surface mediated interactions between carboxylate groups and side C-H bonds of neighboring molecules. However, the N atoms of the pyrazine ring present lone pairs to neighboring sites, which would be mutually repulsive to similarly oriented neighboring molecules. This effectively deactivates self-assembly in the second direction, leading to the one-dimensional chains observed in Figure 1.
Upon subsequent deposition of Cr to the surface, the surface morphology changes dramatically. PDA molecules are now collected into metal-organic complexes on the surface. These appear as bright features on the Cu terraces, as shown in Figure 3. We have shown that the Cr coverage on the surface can be varied in a controlled way, thereby controlling the relative concentration of the pure PDA chains (shown in Figure 1) and the Cr-PDA structures (shown in Figure 3). Analysis of these features is ongoing and holds promise for surface-supported metal-organic complexes.
Figure 3. Scanning tunneling microscopy image of PDA with co-deposited Cr on the Cu(100) surface. This image shows a saturation coverage of Cr so that the pure PDA chain structure has been consumed in the formation of Cr-PDA complexes. Image size is 100 nm x 100 nm.
A key scientific theme in this work is the question of whether metal centers in metal—organic complexes at surfaces can be tuned to the same degree as in solvated molecular complexes. The realization in recent years of highly-ordered surface structures by supramolecular self-assembly, including metal—organic coordination bonding, has opened up many opportunities for efficient and designable surface architectures. Here, the metal centers are in contact with the surface, which will also play a role in the charge state of the metal. This may provide opportunity for further tuning of the active adsorption site.
This project has provided excellent training opportunities for two female graduate students in our chemistry Ph.D. program. Amanda Lear has worked on this project during this funding period and has focused on the development of chemical characterization techniques. She has presented her results and progress at the Central Region meeting of the American Chemical Society (CERMACS), the Turkey Run Analytical Chemistry Conference, as well as department seminars and group meetings. A second graduate student Jennifer Sandahl has had increasing involvement in this project during this funding period. Ms. Sandahl focuses on the high-resolution scanning microscopy studies, some of which are shown in Figures 1 and 3. She has developed skills to prepare these surface structures and image them using state of the art STM capabilities in our laboratory. She has presented the results of her work in a recent symposium sponsored by the National Organization for the Professional Advancement of Black Chemists and Chemical Engineers.