57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR150675-UR1: Planar Chiral Sulfinyl Diene Iron(0) Tricarbonyl Complexes as a Platform for Diastereoselective Synthesis of Spiroketals
Robert S. Paley, PhD, Swarthmore College
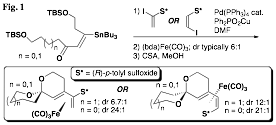 The development of the chemistry of enantiomerically pure sulfinyl diene iron(0) tricarbonyl
complexes has continued during the duration of the grant period. Following our earlier discovery that
appropriately substituted complexes of this type can undergo diastereoselective spiroketalizations
(Fig. 1), our laboratory has been exploring the
substrate scope of this process.
The development of the chemistry of enantiomerically pure sulfinyl diene iron(0) tricarbonyl
complexes has continued during the duration of the grant period. Following our earlier discovery that
appropriately substituted complexes of this type can undergo diastereoselective spiroketalizations
(Fig. 1), our laboratory has been exploring the
substrate scope of this process.
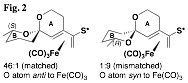
Our initial findings demonstrated that the planar chirality of the substrate was sufficient to influence the formation of the spiroketal stereocenter with a high degree of selectivity. However, it remained unclear whether any additional stereocenters located at positions along either spiroketal ring would play a role in determining the selectivity of the cyclization. While the impact of methyl groups along the two available positions of the A ring was established prior to the grant period, significant effort was next undertaken to complete the study by preparing and inducing the cyclization of substrates with methyl groups along B ring positions. This study is nearly complete at this time, and a clear pattern has emerged that is indicated in the examples shown (Fig. 2). It was found that substrates with "matched" stereocenters (i.e., with methyl groups that would be equatorial) could improve the diastereoselectivity of spiroketalization to as much as 40:1. On the other hand, diastereomeric substrates usually had a preference to invert the spiroketal stereocenter rather than exhibit an axial methyl group. These "mismatched" substrates underwent spirocyclization with little selectivity in some cases, and in others with selectivity as high as 9:1 but dictated by the methyl group's conformational preference to be equatorial rather than by the planar chirality of the iron(0) tricarbonyl complex.
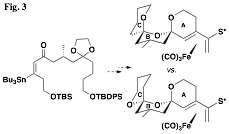 This concept is being extended to the more
challenging bisspiroketal analogs. Spiroketalization
of an unsubstituted precursor gives only a modest
selectivity as all four diastereomers are produced in
a ca. 4:4:1:1 ratio. The stereocenter
at the B/C ring juncture is created without selectivity, likely a result of the
energetically contradictory dipole repulsion (between the oxygen atoms of the B
and C rings) and maintenance of the anomeric
effect. We are currently preparing
substituted analogs with the hope that a single methyl substitutent
on either the B or C ring can tip the balance between these energetic factors
and result in improved stereocontrol (Figs. 3 and 4).
This concept is being extended to the more
challenging bisspiroketal analogs. Spiroketalization
of an unsubstituted precursor gives only a modest
selectivity as all four diastereomers are produced in
a ca. 4:4:1:1 ratio. The stereocenter
at the B/C ring juncture is created without selectivity, likely a result of the
energetically contradictory dipole repulsion (between the oxygen atoms of the B
and C rings) and maintenance of the anomeric
effect. We are currently preparing
substituted analogs with the hope that a single methyl substitutent
on either the B or C ring can tip the balance between these energetic factors
and result in improved stereocontrol (Figs. 3 and 4).
We
have also extended this chemistry to benzannulated
substrates. Our concern that the
fusion of an aromatic system to the B ring – and the resulting flattening
of its chair conformation – would lead to a diminished spiroketalization selectivity was indeed observed. Diastereoselectivity
for the case depicted in Fig. 5 was reduced to a modest 5.5:1. However, addition of a methyl group
capable of occupying a
pseudoequatorial position dramatically enhanced the selectivity to
40:1. 
Finally,
we have initiated a new project aimed at the synthesis of azaspirocyclic
systems. The preparation of a
cyclic ketimine presented a significant challenge
that was met with a strategy that began with a Curtius
rearrangement in order to assemble a suitably protected homopropargylic
amine (1, Fig. 6). The dianion
generated from it reacted exclusively at the alkyne terminus with an assortment
of substituted aldehydes. 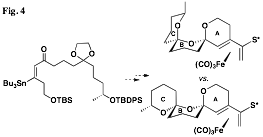 The ketimine was then prepared in a single step by deprotection of the carbamate
and cyclization following a sequence of steps in
accord with our established methodology.
At the present time, reduction and allylation
of this imine have each been performed, each proceeding
with perfect 100:0 diastereoselectivity. We are currently exploring closing the
ring by metathesis, as well as via an aldehyde-imine pinacol
coupling.
The ketimine was then prepared in a single step by deprotection of the carbamate
and cyclization following a sequence of steps in
accord with our established methodology.
At the present time, reduction and allylation
of this imine have each been performed, each proceeding
with perfect 100:0 diastereoselectivity. We are currently exploring closing the
ring by metathesis, as well as via an aldehyde-imine pinacol
coupling.
Three
undergraduate co-workers have benefited from support from the current grant;
two for two summers (directly receiving stipends from the ACS-PRF grant for one
summer each), and one who had received a stipend
from a different source but benefited from available
grant funds for supplies and fine chemicals. This latter student is currently in his
first year of a chemistry doctoral program (Princeton) and the other two, currently
seniors, are beginning their own chemistry graduate school applications at the
present time.
This latter student is currently in his
first year of a chemistry doctoral program (Princeton) and the other two, currently
seniors, are beginning their own chemistry graduate school applications at the
present time.
As Principal Investigator the grant has provided the opportunity for me to reinvigorate a mid-career research program at a time of fiscal belt-tightening and flat departmental budgets that would have had a negative impact on productivity. Furthermore, the supply budget provided by the grant helped to support my recent one-semester sabbatical leave, taken from January to May of 2012 in my own lab at Swarthmore. With the support provided by the grant my co-workers and I expect to have obtained sufficient results to enable the submission of at least two manuscripts for publication by the end of the grant period. I anticipate that these efforts will serve to launch further developments to be supported by federal grant money.










