57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR950161-UR9: Experimental Study of Transport Phenomena of Evaporating Fuel Films
Peter L. Kelly-Zion, PhD, Trinity University
Summary
The overall goal of this project is to experimentally investigate the thermal and mass transport processes controlling the evaporation of sessile hydrocarbon drops. Through systematic experimentation during which the relative influences of the transport processes are controlled, this project is providing a significantly better understanding of the transport behavior involved in the evaporation process.
Research Overview for 2011-2012
Three different sets of experiments were employed, focusing on the role of vapor transport by diffusion and natural convection: measurements of evaporation in air at atmospheric pressure, measurements of evaporation in a variety of gases and pressures, and measurements of the vapor concentration distribution above an evaporating drop.
Measurements in Atmospheric Air
Our previous work has indicated that drop evaporation at room temperature and pressure is controlled by the rate of vapor transport, which occurs by a combination of diffusion and natural convection for the hydrocarbons we study. All of the components we have studied have a molar mass greater than that of air.
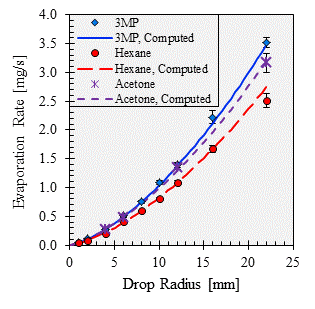
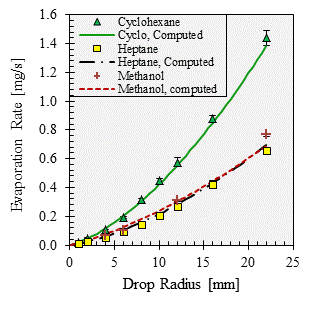
Published correlations that account for both diffusion and natural convection exist for the evaporation of sessile water drops, but there is not a published correlation that is applicable for a broad range of hydrocarbons. Using the fairly large database of evaporation rate data that we have measured, we are working to develop a correlation to compute the evaporation rates of hydrocarbon drops based on the drop size and the physical properties of equilibrium vapor pressure, molar mass, and mass diffusivity. As shown in Figs. 1 and 2, our correlation does a good job of fitting the data, with an RMS error of 5.7%. The influences of diffusion and natural convection seem to be generally captured. For example, while methanol evaporates at a rate approximately equal to that of heptane (shown in Fig. 2), methanol has a much higher diffusivity and much lower vapor pressure and molar mass than heptane has, and consequently methanol's evaporation rate is likely more influenced by diffusion than is heptane's rate.
Figure 1. Measured and computed evaporation rates of 3-methylpentane, hexane, and acetone.
Figure 2. Measured and computed evaporation rates of cyclohexane, heptane, and methanol.
Measurements in a Variety Gases and Pressures
Experiments were conducted in a pressure-vessel to enable the pressure and identity of the ambient gas to be controlled, and thereby to change the relative effects of diffusion and natural convection.
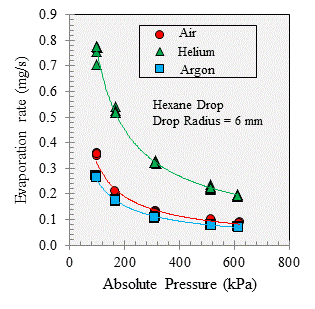
Figure 3 presents the measured evaporation rates for a hexane drop evaporating into air, helium, and argon over a range of pressures. Both the mass diffusivity and the relative density difference (i.e. the difference between the vapor-gas mixture density and that of the ambient gas divided by the ambient gas density) are inversely proportional to pressure. Thus, as pressure increases, both diffusion and natural convection decrease.
Figure 3. The evaporation rate as a function of ambient pressure.
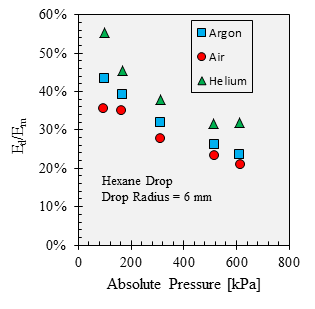
The interesting aspect of these measurements is the relative influence of diffusion and natural convection on the evaporation rate. A solution for diffusion-limited evaporation from a circular disk was used to estimate diffusion's contribution to the overall evaporation rate, and the fraction of the diffusive evaporation is plotted in Fig. 4. This plot indicates that while both the diffusivity and the relative density difference are inversely proportional to pressure, the influences of diffusion and natural convection on the evaporation rate are affected by pressure to different degrees.
Figure 4. Fraction of diffusive transport to the measured evaporation rate of hexane as a function of pressure.
Infrared Spectroscopy Measurements of Vapor Phase Concentration
The third major investigation was the measurement of the vapor concentration distribution above an evaporating drop. Our goal is to obtain data that will enable us to compute the rate of vapor transport by diffusion, which is proportional to the gradient of the concentration. Once the rate of diffusion is computed, the balance between that rate and the measured evaporation rate can be attributed to convection. In this way, the roles of diffusion and convection can be determined quantitatively. This is an experiment that we have been developing for a few years and this year we made important discoveries regarding the limits and repeatability of the measurements.
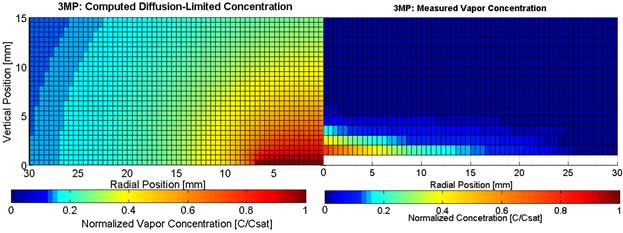
Figure 5 compares vapor distributions above an evaporating drop of 3-methylpentane. The plot on the left presents the computed results for diffusion-controlled evaporation and the plot on the right presents the measured results. Due to axial symmetry, the distribution over half of the drop is presented. The drop radius is 6.5 mm. The measured distribution is much flatter than the nearly hemispherical distribution computed for diffusion-controlled evaporation, which suggests that a downward and radially outward convective flow is occurring.
Figure 5. Comparison of the vapor distribution computed for diffusion-controlled evaporation (left) to the measured distribution for an evaporating drop of 3-methylpentane.
Impact of the Project
This research program continues to have a very large impact on the participating students. During the last year, nine undergraduate students participated in the research and learned valuable lessons not only about the physical phenomena we are studying but, more importantly, about how to go about conducting research, i.e. how to think about problems, how to design and conduct experiments, and how to analyze measurement results. Four of the students devoted 10 weeks during the summer to the research and thereby gained an intensive research experience.
For the principal investigator, this PRF supported research is his primary research focus and, therefore, is very important for his continuous development as a researcher and academician.
In addition to the direct impact that the project has on students involved and the principle investigator, the project is very beneficial to Trinity University's Engineering Science Department, which is small and completely undergraduate. This PRF-sponsored project has the highest profile and supports the largest number of students of the research projects based in Trinity's Engineering Science Department.