57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI1050927-UNI10: Characterizing Synthesis and Ion Transport in Microporous Mixed-Polyhedral Frameworks
Aaron Celestian, PhD, Western Kentucky University
Microporous materials have a long and important history in petroleum science. The ion diffusion properties of natural zeolites and their synthetic analogues have been used successfully for catalysis and molecular separation during the petroleum refinement processes. The goal of this research is to understand processes that direct ion diffusion and allow for specific selectivity in heterosilicate microporous titanium/zirconium/niobium silicate, a class of zeolitic analogues. The tools utilized and insights gained into ion diffusion processes will be broadly applicable to other microporous materials and will directly benefit energy and petroleum sciences.
Zorite, sitinakite, and umbite are microporous heterosilicates that have been used as catalysts in the petroleum industry. Zorite (also known as ETS-4) and sitinakite (also known as TS or CST) are titanium silicates that are naturally found in hydrothermally altered igneous rocks. Umbite is a naturally occurring zirconium trisilicate. The ion exchange characterization of these materials are being performed because of their historical uses, and their potential for high temperature and selective catalysis. The goal of this research is to detail the cation exchange mechanisms of rare earth elements (REEs: Y, Eu, Gd, Tb) and transition metals (Ni, Cu, Zn).
In Situ Time-Resolved Raman
In situ time-resolved Raman microscopy served as the primary tool to determine structural conformational changes during the ion exchange process, revealing polyhedral distortion changes as REE diffuse into the crystal structure. Example plots of REE exchange sitinakite-Na demonstrate that the structural channels become more elliptical as REE cations diffuse into the structure (Fig. 1). These channel distortions were marked by changes in the Si-O-Ti and Ti-O-Ti bending modes in the Raman spectrum as peaks move closer relative to each other (Fig. 1). The difference Raman shifts plots demonstrate that the REEs each induce similar channel distortions, however, the rates and magnitudes of the distortions differ (Fig. 1).
Distortions of the TiO6 polyhedra were apparent in higher wavenumber peaks in the Raman spectra (Fig. 1d, inset). These peaks represent internal stretching modes of the TiO6 and clearly shift as REE cations migrate into the structure. In one case, there may be up to six discrete TiO6 polyhedral changes, and each step has its own kinetic rate law potential and unique chemical functionality. Bonding geometries are currently being modeled via quantum mechanical calculations using Quantum Espresso. A band-gap transition may occur at the arrow in Fig. 1d as a consequence of molecular stresses in the channel, polyhedron, or both. A band-gap transition is also supported by UV-Vis data (not shown). It is unlikely that the band-gap transition is due to a structural symmetry change, because time-resolved XRD indicate a constant space group and minimal unit cell volume changes. A more likely explanation is polyhedral distortions/stresses as the exchanged REE electron orbitals interact with framework cations. This would necessitate that the REE reside close to the framework. X-ray diffraction data that were collected in the summer of 2012 could provide the required evidence for this, and are currently being analyzed.
Surprisingly, no REE exchange occurred in the H-exchanged, activated zorite, sitinakite, and umbite (zorite-H, sitinakite-H, and umbite-H). Activated microporous materials are usually fast ion conductors for both monovalent and divalent cations. The small H cation should easily diffuse out of the crystal structure in favor of a larger, higher valance cation. However, the REEs are completely rejected by the H-forms of the titanium silicates and zirconium silicates. Our working hypothesis is that it may be a function the overall hydrogen-bond network in the channels and void spaces within the framework. In the as synthesized forms, Na and K ions are shielded by hydration spheres while they are in the channels, and therefore any exchanging cation would not directly interact with those host cations, allowing ion exchange to proceed. In the activated forms, H is bound to the framework, and is not strongly hydrated. The orientation of H2O groups are inverted in the H-forms as compared to the Na- or K-forms of the silicates. High valance cations might be repelled by the covalently bound H on the framework, and therefore would not enter into the crystal structure unless the H2O groups invert. The large Cs cation can force this inversion of the H2O groups, however the REEs are much smaller in size.
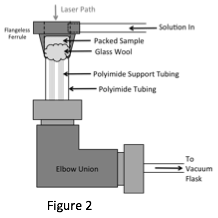
A new ion exchange cell was developed to perform these in situ time-resolved studies. This cell is inexpensive, easy to construct and assemble, and flexible in design to accommodate a wide array of microscope geometries. The main advantage of this cell is that there is very little time in which the laser must pass through liquid, and therefore it is possible to use lasers other than 780nmto perform time resolved analysis using liquids. Fig. 2 shows a schematic of the cell design.
Optimized and Exploratory Synthesis
To explore the interaction of the exchanged REE and
framework cations, experiments are underway to substitute some of the framework
Ti or Zr octahedral cations with REEs, such as Y. One successfully synthesized
Y-doped gaidonnayite-Na compound has been produced as
a micro-crystalline material (crystals < 2 µm3)
and its structure is currently under investigation with high resolution powder
XRD. In addition to exploratory synthesis, we have optimized the synthesis for both zorite, sitinakite, and umbite
using only one reaction vessel for each material, and using relatively less
hazardous reagents. This not only
makes the synthesis safer, but also reduces the amount of expensive reagents
that are used
 and disposed of before and after
the synthesis.
and disposed of before and after
the synthesis.
Outcomes and Student Impacts
Two undergraduate students have been supported with this grant, and have performed the bulk of the experimental work. They synthesized all the materials used in this study, collected data on the Raman microscope and the X7B beamline at the NSLS, and have presented their findings at regional and national conferences. One manuscript on this work is currently in review.











