57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI351009-DNI3: Palladium(I) and Nickel(I) Bridging Allyl Dimers for the Catalytic Functionalization of Carbon Dioxide
Nilay Hazari, PhD, Yale University
Complexes containing Pd allyl moieties are crucial intermediates in a wide variety of important organic reactions, such as allylic alkylation, the telomerization of conjugated dienes and the electrophilic substitution of aldehydes, imines and Michael acceptors. As a result the chemistry of monomeric Pd(II) allyls is well understood and it is accepted that h1-allyls are nucleophilic and h3-allyls are electrophilic. In contrast, despite the synthesis of many Pd(I) dimers with bridging allyl ligands, there have been relatively few studies of the reactivity of these systems and the electronic structure is not thoroughly understood. However, in the last ten years it has become apparent that Pd(I) dimers containing bridging allyl ligands also play an important role in many organic transformations. For example, in 2004, Milstein and co-workers used a Pd(I) dimer with one bridging allyl and one bridging chloride ligand as a precatalyst for the Suzuki-Miyaura coupling. Subsequently, Moore et al. reported the use of related species for the Songashira coupling, and Colacot and co-workers described that Pd(I) dimers were active precatalysts for the arylation of enolates and amines using aryl bromides and chlorides. Recently, our group described a family of unusual Pd(I) dimers with two bridging allyl ligands, which were catalysts for the carboxylation of allylstannanes and allylboranes using carbon dioxide.
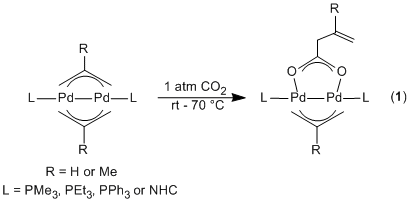
 Our studies this year commenced by
investiga ting the mechanism by which bridging allyl dimers react with carbon
dioxide (Equation 1), using both experimental and computational techniques. The results of our investigation suggest that carbon
dioxide insertion can occur via two different pathways. In one pathway the
bridging allyl ligand acts like a nucleophile and attacks electrophilic carbon
dioxide. This is similar to the pathway through which carbon dioxide inserts
into monomeric Pd and Ni h1-allyls.
Our calculations indicate that the HOMO of the bridging allyl dimers is
localized on the terminal carbon atoms of the bridging allyl ligands. In the
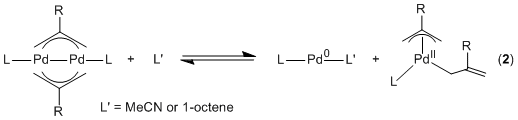
second pathway, which only occurs in the presence of a weakly coordinating
ligand, the bis(allyl) dimer splits into a bis(allyl)Pd(II)(L)
complex and a Pd(0) species stabilized by the weakly coordinating ligand
(Equation 2). The bis(allyl)Pd(L) species then reacts with carbon dioxide,
before recombining with the Pd(0) species to form the dimeric product. Thus, in
the specific case of carbon dioxide insertion reactions, bis(allyl) dimers can
either act as a source of h1-Pd allyls in the presence of weakly coordinating
ligands or as analogs of h1-Pd
allyls in the absence of coordinating ligands.
Our studies this year commenced by
investiga ting the mechanism by which bridging allyl dimers react with carbon
dioxide (Equation 1), using both experimental and computational techniques. The results of our investigation suggest that carbon
dioxide insertion can occur via two different pathways. In one pathway the
bridging allyl ligand acts like a nucleophile and attacks electrophilic carbon
dioxide. This is similar to the pathway through which carbon dioxide inserts
into monomeric Pd and Ni h1-allyls.
Our calculations indicate that the HOMO of the bridging allyl dimers is
localized on the terminal carbon atoms of the bridging allyl ligands. In the
second pathway, which only occurs in the presence of a weakly coordinating
ligand, the bis(allyl) dimer splits into a bis(allyl)Pd(II)(L)
complex and a Pd(0) species stabilized by the weakly coordinating ligand
(Equation 2). The bis(allyl)Pd(L) species then reacts with carbon dioxide,
before recombining with the Pd(0) species to form the dimeric product. Thus, in
the specific case of carbon dioxide insertion reactions, bis(allyl) dimers can
either act as a source of h1-Pd allyls in the presence of weakly coordinating
ligands or as analogs of h1-Pd
allyls in the absence of coordinating ligands.
Interestingly, our studies showed that although species with two bridging allyl ligands react with carbon dioxide, complexes with one bridging allyl ligand and one bridging chloride ligand do not react with carbon dioxide. Density functional theory (DFT) calculations showed that the nature of the HOMO was different between the two types of dimers. In order, to experimentally confirm this difference in the nature of the HOMO, we synthesized Pd(I) dimers with two bridging allyl ligands and one bridging allyl and one bridging chloride ligand, which contain anionic phosphine ligands as the supportling ligand. The photoelectron spectra (PES) of these complexes has been recorded (in collaboration with Professor Lai-Shang Wang at Brown University) and indicate that there is a low energy ionization present in complexes with two bridging allyl ligands, which is absent in species with a bridging chloride ligand. This is consistent with our model for how carbon dioxide insertion into dimers occurs.
The disproportionation proposed in Equation 2 suggests that coordinatively unsaturated monomeric Pd(0) species can be accessed from Pd(I) dimers with two bridging allyl ligands. Given the relevance of Pd(0) species to Pd catalyzed cross-coupling reactions, we were interested in unequivocally establishing that the disproportionation of Pd(I) dimers was occurring and testing whether they would generate an active catalyst for cross-coupling. A series of trapping and cross-over experiments provided strong evidence for the disproportionation. It also appears that complexes with one bridging allyl and one bridging chloride ligand also undergo disproportionation. In fact, we believe that in many cases complexes with bridging allyl ligands may be the resting state of highly active catalysts for Pd catalyzed cross-coupling. Our preliminary experiments indicate that species with bridging allyl ligands are highly active catalysts for the Suzuki-Miyaura reaction. We will continue to pursue these studies over the next year and also look to develop new catalytic reactions which involve Pd(I) dimers.
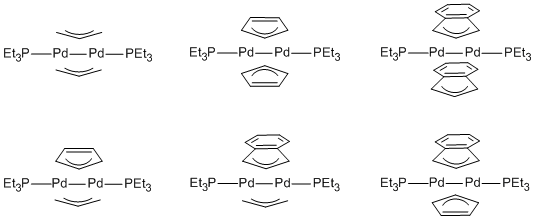 It
has previously been postulated that Pd(I) dimers with bridging cyclopentadienyl
(Cp) or indenyl ligands are analogous to those with bridging allyl ligands,
however the reactivity of these species has not been studied in detail. In
fact, examples of such complexes are scarce as most synthetic routes involve
highly reactive intermediates. Recently, we have synthesized the family of Pd(I)
dimers, shown in Figure 1, which contain bridging allyl, Cp or indenyl and are
all supported by the same ancillary ligand, PEt3. The geometric
parameters determined from X-ray crystallography have been compared for the
different structures and reveal similarities in the bonding between bridging
allyl, Cp and indenyl ligands. The electronic structures of all six
dimers have been investigated using density functional theory (DFT)
calculations. Our calculations indicate that the type of bridging ligand has a
considerable effect on the nature of the HOMO. We believe that understanding the
nature of the HOMO is crucial for predicting the reactivity of these systems
and have performed experimental studies using lutidinium chloride as an
electrophile, which support our computational results. Over the next year
further work will look to explore the reactivity of these systems in more
detail and also use PES to experimentally validate our calculated molecular
orbital diagram. It is also plausible that Pd(I) dimers with bridging Cp and
indenyl ligands are in equilibrium with Pd(0) and Pd(II) complexes and we will
perform trapping and crossover experiments to determine if disproportionation
is occurring. This may lead to new catalytic reactions involving these systems.
It
has previously been postulated that Pd(I) dimers with bridging cyclopentadienyl
(Cp) or indenyl ligands are analogous to those with bridging allyl ligands,
however the reactivity of these species has not been studied in detail. In
fact, examples of such complexes are scarce as most synthetic routes involve
highly reactive intermediates. Recently, we have synthesized the family of Pd(I)
dimers, shown in Figure 1, which contain bridging allyl, Cp or indenyl and are
all supported by the same ancillary ligand, PEt3. The geometric
parameters determined from X-ray crystallography have been compared for the
different structures and reveal similarities in the bonding between bridging
allyl, Cp and indenyl ligands. The electronic structures of all six
dimers have been investigated using density functional theory (DFT)
calculations. Our calculations indicate that the type of bridging ligand has a
considerable effect on the nature of the HOMO. We believe that understanding the
nature of the HOMO is crucial for predicting the reactivity of these systems
and have performed experimental studies using lutidinium chloride as an
electrophile, which support our computational results. Over the next year
further work will look to explore the reactivity of these systems in more
detail and also use PES to experimentally validate our calculated molecular
orbital diagram. It is also plausible that Pd(I) dimers with bridging Cp and
indenyl ligands are in equilibrium with Pd(0) and Pd(II) complexes and we will
perform trapping and crossover experiments to determine if disproportionation
is occurring. This may lead to new catalytic reactions involving these systems.










