57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI451008-UNI4: Specific Ion Effects on the Interfacial Properties at the Hydrophobic/Aqueous Interfaces
Yanjie Zhang, PhD, James Madison University
The Hofmeister series ranks the relative influence of ions on the physical behavior of a wide variety of aqueous processes. This behavior is more pronounced for anions than cations and is quite general. The typical order for the anion series is as follows:
CO32- > SO42- > S2O32- > H2PO4- > F- > Cl- > Br- ~ NO3-> I- > SCN-
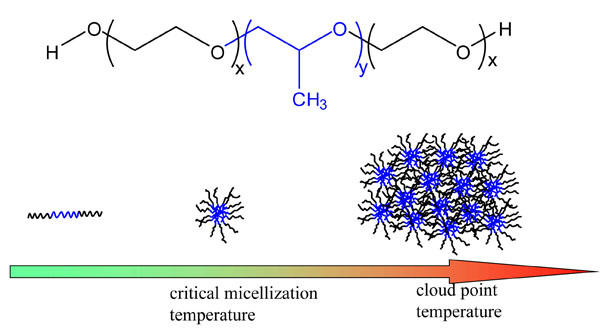
Although the Hofmeister series is a general recurring trend in aqueous solutions, the molecular-level mechanisms of the Hofmeister effects have remained elusive for over 120 years. During the initial grant period, we use a simple model, the temperature-induced phase behaviors of poly-(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) (PEO-PPO-PEO), to further explore the nature of polymer-anion interactions. PEO-PPO-PEO block copolymers are thermoresponsive and the aqueous solutions of the copolymers go through phase transition in two steps as a function of temperature as represented in Figure 1. At low temperatures, PEO-PPO-PEO chains exist as unimers in a dilute solution. When the solution temperature reaches the critical micellization temperature of the polymer, the chains aggregate into micelles with hydrophobic PPO blocks as the core and hydrophilic PEO blocks as the corona. When the temperature increases further and goes through the cloud point, the PEO blocks become dehydrated, inducing the aggregation of micelles. These phase transitions lead to aggregate formation, turning the transparent solution cloudy. We employ an automated melting point system to measure the phase transition temperatures of L44 in the presence of a series of inorganic salts, NaSCN, NaI, NaBr, NaNO3, NaCl, NaF, NaH2PO4, Na2S2O3, Na2SO4, and Na2CO3. We found that chaotropic and kosmotropic anions affect the phase transition of the polymer through separate mechanisms.
Figure 1. Chemical structure and the phase behaviors of PEO-PPO-PEO as a function of temperature.
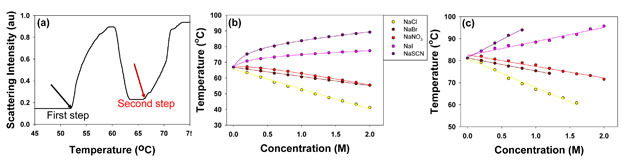
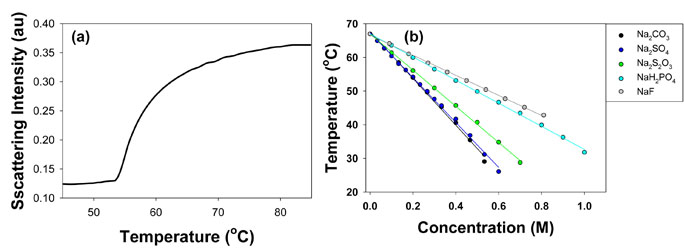
In solutions containing chaotropic anions, L44 showed distinct two phase transitions as illustrated by 1M NaCl in Figure 2a. The phase transition temperature as a function of salt concentration for five chaotropic anions used in this study is shown in Figure 2b and 2c. The first phase transition happens at lower temperature and represents the critical micellization temperature of L44, and the second phase transition indicates its cloud point temperature. In the first step, the chaotropic anions show a nonlinear dependence of phase transition temperature on the anion concentration. This is most obvious for more chaotropic anions such as SCN-, I-, and NO3-. On the other hand, the second step shows a linear dependence on salt concentration. When kosmotropic anions are introduced into the polymer solution, only one phase transition was observed as shown in 0.4 M NaF in Figure 3a. Phase transition temperature decreases linearly with increasing anion concentration for all the kosmotropic anions investigated, including F-, H2PO4-, S2O32-, SO42-, and CO32- (Figure 3b).
Figure 2. (a) Light scattering intensity of L44 solution in 1M NaCl. (b) The first step of the phase transition for L44 against anion concentration. (c) The second step of the phase transition for L44 against anion concentration.
Figure 3. (a) Light scattering intensity of L44 in 0.4 M NaF. (b) Phase transition temperatures for PEO-PPO-PEO against salt concentration for kosmotropic anions.
A mechanism to explain the modulation of the phase transition temperature of L44 in the presence of salts involved interactions of ions with the polymer is proposed. First, anion could change the interfacial tension at the polymer/aqueous interface and further affect the hydrophobic hydration of the polymer. Second, anions could bind directly to the hydrophobic moieties of the polymer. If an anion can shed its hydration water and bind to the hydrophobic portion of the polymer, it will add charge to the hydrated polymer and increase solubility of the polymer. Third, anions can interact with the polymer by changing the entropy of water molecules around the hydrophilic portion of the polymer. Both interfacial tension effect at hydrophobic/aqueous interface and entropy effect at hydrophilic surfaces should be linearly dependent on anion concentration. Anion binding to a hydrophobic surface is a saturation phenomenon. Based on these facts, we modeled our data by a mathematical equation and correlate the fitting parameters to the properties of anions. We found that chaotropic anions worked through changing the interfacial tension at the polymer/aqueous interface and in enhancing the polymer hydration by ion binding. In contrast, the phase transition of the polymer in the presence of kosmotropic anions correlated directly to the hydration entropy of the anions. As a consequence, the polymer showed a two-step phase transition in solutions containing chaotropic anions while displayed a single-step phase transition in the presence of kosmotropic anions. Our results have been published in Langmuir.