57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR150086-UR1: Oxidation of Alkenes in Aqueous Solvent Mixtures Using Environmentally Benign Reagents
Thottumkara K. Vinod, Western Illinois University
Continued research efforts in our laboratory are aimed at developing green chemistry protocols and substitutes for well-known oxidative transformations in synthetic organic chemistry. Water-soluble derivatives of o-iodoxybenzoic acid (IBX) first synthesized in our laboratories have proven to be promising candidates as oxidants for eco-friendly synthetic transformations in water and other aqueous solvent mixtures. Recently we have also reported the catalytic use of such benign reagents for oxidation of alcohols and active C-H bonds in hydrocarbons.
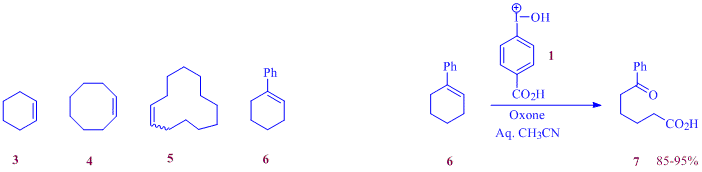
Last year we designed and developed a convenient protocol for the oxidative cleavage of alkenes using the in-situ generated iodonium ions, 1 or 2 obtained from the oxidation of 4-iodobenzoic acid (4-IBAcid) and iodobenzene respectively with Oxone. We also delineated the mechanism of this oxidative cleavage. An extensive substrate scope investigation accompanied this preliminary investigation.
During the current project year our efforts in this area has been centered around the development of a green chemistry experiment for oxidative cleavage of alkenes to be adopted into our undergraduate curriculum and for future dissemination for adaptation by other schools as well. At the outset of this optimization study, several important reaction parameters and criteria were established to be addressed. Cost of reagents and substrates used, length of the reaction time, and ease of product characterization and instructional value of the methods used etc. are important among them.
Though several cheap and common alkene susbstrates (cyclohexene, cyclooctene, cyclododecene) were initially tried as susbstrates,reasonable yields of the cleaved products could not be obtained from the cleavage reaction during a 2-3 h reaction, forcing us to use 1-phenyl-1-cyclohexene, 6 as the most suitable substrate for this project. The selection of this substrate was made hesitantly due to the cost of the alkene ($41.60/5 g), however we felt that benefits from the successful optimization and demonstration of the experiment supersedes the higher cost. The oxidative cleavage of 1-phenyl-1-cyclohexene, 6 was carried out using the in-situ generated iodonium ion, 1 from commercially available 4IBAcid and Oxone. Extensive optimization studies were carried out by varying the molar ratio of 4IBAcid, and the solvent ratio of water to acetonitrile (v/v). A variety of eco-friendly co-oxidants were also tried to find a replacement for the acidic Oxone. A serious drawback of Oxone as a co-oxidant used in our original paper and for the optimized undergraduate green experiment is the acidity of the co-oxidant making the new protocol unsuitable for the cleavage of alkenes with acid labile groups. During the past year we have also initiated an investigation to identify a non-acidic co-oxidant and this investigation has led us to the use of K2S2O8 in buffered reaction media.
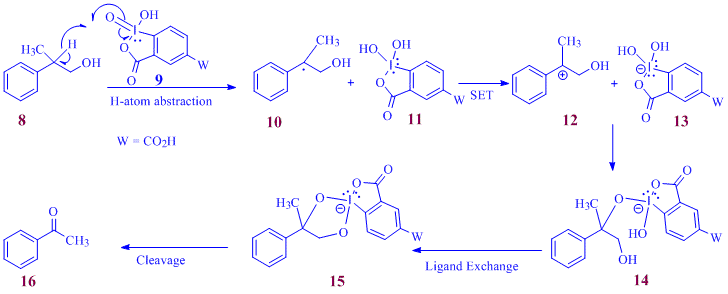
Solvent dependant oxidation behavior of alcohols was observed during a related investigation in which water-soluble IBX derivative 9 is employed as a green oxidant. At the outset of this investigation we were aware of the fact that the mechanism of oxidation of alcohols in aqueous solvent mixtures using this reagent does not follow the well accepted ligand-exchange mechanism as this mechanism involves fast and reversible alcohol coordination onto the iodine center with the concomitant elimination of a water molecule.
Scheme 1: Mechanistic basis for the hypothesis leading to the Cα-Cβ cleavage in select alcohols
The currently postulated mechanism of oxidation of alcohols using the water soluble reagent 9, and other derivativesthereof, involve a rate determining αH atom abstraction clearly explaining the observed differences in the rate and ease of oxidation of the various alcohols based on the bond dissociation energies (BDE) of the pertinent αC-H bond. During the current project period we speculated that the water-soluble IBX derivatives may be capable of cleaving Cα-Cβ bond in alcohols provided there is an abstractable hydrogen atom on the α carbon (i.e. a C-H bond with a low BDE). Mechanistically this hypothesis is explained in the scheme above. An enthalpically favored H-abstraction from the benzylic carbon and a subsequent single electron transfer (SET) to a highly oxygenated iodine center leads to the cyclic iododiester intermediate, 15, the decomposition of which then leads to the cleaved product(s). Preliminary results from the attempted cleavage of 2-methyl-2-phenylpropan-1-ol, 8 using 2.4 equiv. of 9 in aqueous acetonitrile provided a mixture of acetophenone,16, α-hydroxyaldehyde, 17 and the aldehyde, 18 in 27%, 26% and 27% respectively.
Scheme 2: Reductive decomposition pathways for 15
The formation of the first two products can be explained from the formation and the subsequent decomposition of the cyclic iododiester intermediate 15, as shown in Scheme 2. Efforts are currently being directed to optimize theconditions for an effective cleavage of the C?-C? bonds of alcohols that bear a phenyl group on the ? carbon simultaneously placing an abstractable hydrogen atom on the α-carbon.
Oxidation of elemental iodine to I+ using bis(trifluoroacetoxy)iodobenzene (BTI), 19 and the subsequent use of the in-situ generated 20 as the electrophilic iodine source in co-iodination of electron deficient alkenes are currently underway in our laboratory. Treatment of cinnamic acid, 21, with BTI in presence of
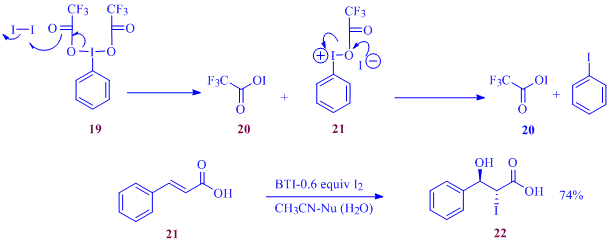 Scheme 3: Iodine atom economic co-iodination
of alkenes using BTI/I2/Nucleophile
Scheme 3: Iodine atom economic co-iodination
of alkenes using BTI/I2/Nucleophile
0.6 equiv. of elemental iodine and a water as the nucleophile provided a 70% yield of 22, indicating the enhanced electrophilicity of CF3CO2I. An expanded substrate scope investigation is currently underway.
In summary, we continue to make progress in many areas of the originally proposed research. Manuscripts describing the results from the solvent dependant oxidation studies and the green chemistry experiments developed for implementation in undergraduate curriculum are currently being prepared. Both undergraduate and graduate students (MS) continue to get trained in all aspects of chemical research through this funded proposal.