57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND450709-ND4: Development of N-Aryl-2,3-Naphthalimide Dyes for White-Light Emission with Applications Toward White Organic Light-Emitting (WOLEDs)
Michael D. Heagy, PhD, New Mexico Institute of Mining & Technology
Introduction and Background
Organic light emitting diodes (OLEDs) have gained attention as one of the most appealing solutions for low energy consumption in solid-state lighting.[1] To date organic white light-emitting devices (WOLEDs) are obtained by combining the emission from red, green and blue or sky-blue and orange emitters. The combination of these emitters can be achieved by the deposition of multiple layers on top of each other,[2] by mixing them into one single emitting layer,[3] combinations of the two techniques,[4] and by combining them into polymeric structures.[5] Contrary to polymers, small molecules can be evaporated and therefore very complex multi-layer structures can be constructed. This high flexibility in layer design is the main reason for the high efficiencies of the small-molecule (SM)-OLEDS. These approaches require more complex device architectures and production processes compared to single-emitter based OLEDs, which has so far greatly hindered their market entry. Therefore, development of a white light-emitting single molecule is very much desired. Multinuclear complexes[6] or excimers[7] have been designed for such a purpose. However, despite being single molecules, those approaches still rely on the combination of two emitting centers, which is expected to show color drift over time. Fluorophores that emit white light may potentially simplify and lower the cost of devices such as WOLEDs,[8] flat panel displays[9] and electronic paper displays.[10] Synthetic organic fluorophores have at times been criticized for wide band emission. However, broad visible region bands promote panchromatic emission and improve the color rendering of white light. [11]
Our group has gained considerable insights into the area of new fluorophore design. Specifically, in the area of fluorescent chemosensor development, we have designed a number of dual fluorescent dyes that display ratiometric detection of biologically relevant analytes.[12] [13][14] Our efforts into the development of N-aryl-1,8-naphthalic dicarboximides (NI) as dual fluorescent (DF) dyes for biomedical applications has provided new insights into the photophysical processes responsible for two-color emission.[15] The new direction we have taken with these versatile fluorescent platforms leads toward extending the dual fluorescent features of these systems to more panchromatic or white light emission. Plans to accomplish this are centered on the transition from our 1,8-naphthalimide fluorescent system to the structurally and symmetry related 2,3-naphthalimide class of fluorophores. Based on our current results for the 2,3-naphthalimide dyes, this report outlines (1) synthetic procedures accomplished for pachromatic 2,3-napthalimide dyes (2) recent photophysical results, specifically demonstrating the panchromatic emission of an newly prepared 2,3-naphthalimide and finally (3) updates on our studies/acquired equipment necessary for the electroluminescent measurements of these dyes as potential WOLED systems.
Synthetic results since funded: Synthesis of 2,3-NI dyes as potential WOLEDs
According to our photophysical model from symmetrically related 1,8-NI's, a synthesis that generates various 5- and 6-substituted 2,3-naphthalic anhydrides bearing electron-withdrawing substituents is critical for new WOLED design. 2,3-naphthalic anhydrides can be obtained by different synthetic routes, most notably Diels-Alder, thereby affording a wide variety to be synthesized.
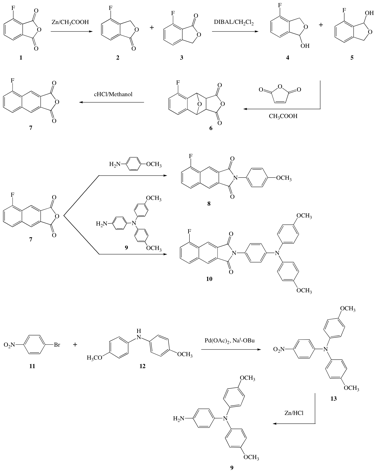
Graduate student Lili Bao has completed the synthesis of 5-fluoro-2,3-napthalic anhydride 7, with the following scheme 1 shown below. This anhydride has been coupled to both p-anisidine to give dye 8 and 4-aminophenyl 4,4'-dimethoxyldiphenylamine to give dye 10.
SCHEME 1. Completed synthetic routes to 5-fluoro-2,3-NI 8 and 10
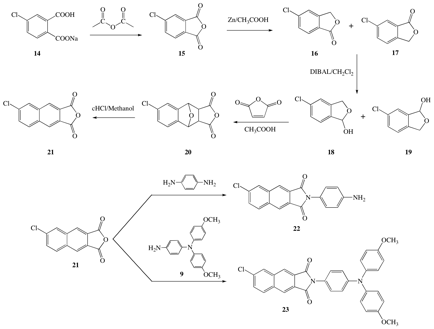
In scheme 2, 6-chloro-2,3-naphthalic anhydride is prepared in a similar manner. This anhydride was coupled to diaminophenylene to deliver dye 22 and with 4-aminophenyl 4,4'-dimethoxyldiphenylamine to generate 23.
SCHEME 2. Completed synthesis of 6-chloro-2,3-NI derivatives 22 and 23
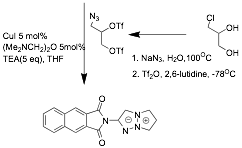
In addition to the dye systems shown above, graduate student Ranjith Meka has completed a synthetic route to naphthalene yne-imides shown in scheme 3. These precursors can be used in the click-reaction of Huisgen reaction whereby a new chromophore group is formed via triazole formation.
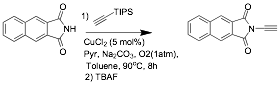 It is anticipated that these yne-imides will
open a new route to the N-penatalene system shown
below as another class of panchromatic dyes.
It is anticipated that these yne-imides will
open a new route to the N-penatalene system shown
below as another class of panchromatic dyes.
Current spectroscopic results
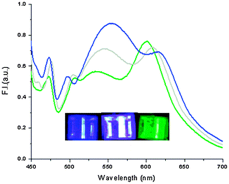
Our first set of steady state solution fluorescence results is shown as a panchromatic spectrum with new dye 10. Our next step includes optical and fluorescence characterization of the other afore-mentioned dyes prior to thermal deposition of these dyes onto ITO sample slides.
Figure 1. The emission spectra of 20 μM compound I in water with 0.10% DMSO at different excitation wavelengths 390 nm, 423 nm and 450 nm, corresponding to blue, white and green photographs, respectively. Colored light of cuvette is due to output of excitation light
Electroluminescence measurements
| |

The final phase of the funded project involves application of the dyes toward developing simple light emitting diodes. In April 2012, our acquired an ultra high vacuum system manufactured by the MBraun company (shown to the left). We have also purchased an Avantes electroluminescence instrument and Kiethley 2400 source meter coupled to a pico-amperometer using a luminance meter to calibrate the photocurrent.
References
[1] Gustafsson, G.; Gao, T.; Treacy, G.M.; Klavetter, F.; Colaneri, N.; Heeger, A.J. "Flexible light-emittin-diodes made from soluble conducting polymers" Nature, 1992, 357, 477-479. (a) Bulovic, V.; Gu, G.; Burrows, P.E.; Forrest, S.R.; Thompson, M.E. "Transparent light emitting diodes", Nature, 1996, 380, 28-29.
[2] D'Andrade, B.W.; Thompson, M.E.; Forrest, S.R.; "Controlling exciton diffusion in multilayer white phosphorescent organic light emitting devices" Adv. Mater. 2002, 14, 147-151.
[3] D' Andrade, B.W.; Holmes, R.J.; Forrest, S.R.; "Efficient organic electrophosphorescent white-light-emitting device with a triple doped emissive layer" Adv. Mater., 2004, 19, 3599-3602.
[4] Sun, Y.; Giebink, N.C.; Kanno, H.; Ma, B.; Thompson, M.E. Forrest, S.R.; "Management of singlet and triplet excitons for efficient white organic light-emitting devices" Nature, 2006, 440, 908-912.
[5] Luo, J.; Li, X.; Hou, Q.; Peng, J.; Wang, W.; Cao, Y. "High-efficiency white-light emission from a single copolymer: Fluorescent blue, green, and red chromophores on a conjugated polymer backbone" Adv. Mater., 2007, 19, 1113-1117.