57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI1050631-DNI10: Novel Nanocomposite Materials for Efficient Photocatalytic Reduction of CO2 to Fuels
Ying Li, University of Wisconsin (Milwaukee)
Objective
Photocatalytic reduction of CO2 with water by sunlight provides a new opportunity for simultaneous mitigation of greenhouse gases and production of energy-bearing compounds that can be further converted to substitutes for petroleum products. However, a low CO2 conversion rate and the lack of efficient and inexpensive catalysts have impeded the development of this technology. The objective of this research was and to design novel nanostructured composite materials to achieve high CO2-to-fuels conversion efficiencies and to understand the mechanism of CO2 photoreduction with water.
Results
Over the past one and a half years, we have achieved significant progress towards realization of the project goal. Specifically, we have developed three novel materials for CO2 photoreduction: (1) Cu-modified TiO2 nanoparticles with engineered surface defect sites; (2) Ag-deposited TiO2 nanoparticles assembled to form porous microspheres; (3) Ce-doped TiO2 dispersed on ordered mesoporous SBA-15 support. All three novel catalysts have demonstrated much more effective activities in CO2 photoreduction to fuels compared with bare TiO2 nanoparticles.
(1) Surface defective Cu/TiO2 nanoparticles
Cu/TiO2 with surface defect sites, Cu(I)/TiO2-x, was prepared by a simple precipitation method followed by heat treatment in an inert gas environment (e.g., helium) at moderate temperature (e.g. 300 °C). In situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) identified the surface defect sites to be Ti3+ and oxygen vacancies, and Cu(I) dominated the Cu species on TiO2. The in situ DRIFTS analyses also demonstrated that CO2- species, generated upon an electron attachment to CO2, are spontaneously dissociated into CO even in the dark over the Cu(I)/TiO2-x catalyst. This dissociation process is associated with the oxygen vacancies that not only provide the electron centers but also the sites for the adsorption of oxygen atom from CO2. Isotopic carbon-labeling experiments confirmed that the produced CO is indeed derived from CO2 (see Figure 1). Compared with in the dark, CO2 activation and dissociation under photo-illumination is remarkably improved, possibly due to sustained electron supply and partial regeneration of surface oxygen vacancy induced by irradiation. The results from DRIFTS analyses were verified by the measurement of catalytic activity using gas chromatography (GC). These findings are important to advancing the understanding in the chemistry of CO2 adsorption and photocatalytic reduction on the surface of metal oxide catalysts.
(2) Porous microspheres assembled by Ag/TiO2 nanoparticles
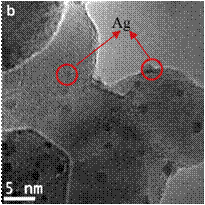
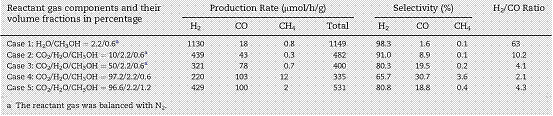
A simple ultrasonic spray pyrolysis method was used to prepare mesoporous Ag/TiO2 composite in the morphology of microspheres using TiO2 (P25) and AgNO3 as the precursors. Ag nanoparticles in the size of 1-2 nm were deposited on TiO2 (see Figure 2). The activity of the Ag/TiO2 catalyst was measured for solar fuel production from CO2 photoreduction with H2O in the presence of methanol as a hole scavenger. CO and H2 were the major products from the photocatalytic reactions. The optimal Ag concentration on TiO2 was 2 wt%. The H2 production rate of the 2% Ag/TiO2 prepared by the spray pyrolysis method exhibited a six-fold enhancement compared with that prepared by a conventional wet-impregnation method. The ratio of H2/CO (in the range from 2 to 10) can be controlled by adjusting the ratio of reactants (see Table 1). This controllable H2/CO ratio in the reaction products and negligible CH4 production indicates that it is a promising technology to produce syngas (the mixture of H2 and CO) through photocatalytic CO2 reduction on the engineered Ag/TiO2 samples.
(3) Ce-doped TiO2 dispersed on SBA-15
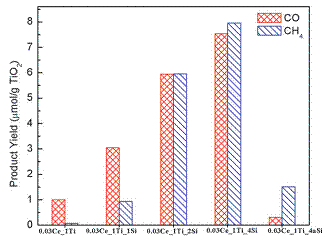
Ce-TiO2 and Ce-TiO2/SBA-15 composites were prepared by a sol-gel method and tested for CO2 photoreduction with water. Compared with pristine TiO2, TiO2 doped by 1 or 3% Ce improved the production of CO by four times. The reason may be due to the facilitated charge transfer induced by the doped Ce ions, the higher surface area of the catalyst, as well as the stabilization of anatase phase. Dispersing Ce-TiO2 nanoparticles on the mesoporous SBA-15 support further enhanced both CO and CH4 production (see Figure 3). Particularly the CH4 production was enhanced by up to 115 times compared with unsupported Ce-TiO2. The superior catalytic activity may be related to the partially embedded Ce-TiO2 in the ordered 1-D pores in SBA-15 that form synergies between the different components and enhance the diffusion and adsorption of CO2. This mechanism also correlates well the results that using SBA-15 as the support led to more than 10 times higher activity in CO2 photoreduction than using amorphous silica as the support.
Future Plan
In the second year of the project, research efforts will be dedicated to further improving the overall CO2 conversion rate and control of product selectivity. Cu/TiO2 catalysts with controllable Cu oxidation state (Cu0, Cu+, or Cu2+) will be synthesized and the effect of the different Cu species will be examined by combined GC/MS measurement of catalytic activity and in situ DRIFTS study. Nonmetal doping in the TiO2 will be engineered to enhance visible light activity.
Impact
So far, the research supported by ACS-PRF has resulted in three journal articles published in Journal of Physical Chemistry C, International Journal of Hydrogen Energy, and Catalysis Science & Technology, respectively. The results were also disseminated in several national conferences. The ACS-PRF project has trained one postdoc and one graduate student. It also provided research experience for one undergraduate student who was co-author of one of the journal publications. The ACS-PRF grant has served as important seed funding for the PI to secure other funding to conduct research in the field of renewable energy.
Figure 1. In situ DRIFT spectra for 13CO2 interaction with defective Cu(I)/TiO2-x in the dark
Figure 2. SEM and HRTEM images of the Ag/TiO2 catalyst
Figure 3. Production of CO and CH4 over Ce-TiO2/SBA-15 catalysts under UV-vis illumination for 4 h
Table 1. Production rate and product selectivity of the 2%Ag/TiO2 sample under simulated solar illumination