57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR149493-UR1: Development of Enantioselective Nitrogen Arylation Reactions
Russell D. Viirre, PhD, Ryerson University
The development of new reaction methodologies capable of enantioselectively forming chiral products from achiral starting materials is an area of intense research. When the selectivity in such reactions is induced from only a very small amount of chiral catalyst, the methodology can be a cost-effective way to form very valuable chiral products from relatively inexpensive starting materials. With this goal in mind, we have been exploring the utility of the Buchwald-Hartwig reaction as an enantioselective reaction.
In general, a Buchwald-Hartwig reaction is a palladium-catalyzed cross-coupling reaction between a nitrogen nucleophile, and an aryl halide, forming an aromatic C-N bond. While the literature contains hundreds references to this important reaction, fewer than ten studies have explored the possibility of using a Buchwald-Hartwig reaction to induce asymmetry in a product, and most of these achieved only modest selectivities.
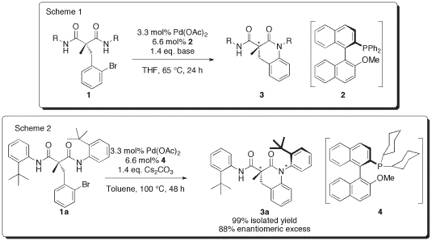
In the first year of this ACS PRF Undergraduate grant, we developed and optimized the enantioselective ring-closing Buchwald-Hartwig reaction of symmetrical malonamides 1 (Scheme 1). Under the optimized conditions, employing the chiral phosphine ligand (R)-MOP (2), the cyclized products 3 are obtained in excellent yields and up to 96% ee. In the second year of this grant, we have developed a synthesis for the related dicyclohexylphosphine ligand 4, and shown that it is the ideal supporting ligand for the asymmetric intramolecular Buchwald-Hartwig reaction of 2a (Scheme 2). This formation of 3a is notable since the product contains both a chiral center, as well as a chiral axis (due to blocked rotation about the nitrogen to o-t-butylphenyl bond). In this case, from achiral starting material 2a, only a single diastereomer is formed in 99% yield and 88% ee. The configuration of the major enantiomer was confirmed by X-ray crystallography. In the third year of this grant (the subject of this report), we began studies to help us understand the origin of diastereoselectivity in the reaction shown in Scheme 2. We also made significant headway in developing palladium complexes with ligands such as 2 and 4, which we hope will serve as efficient standalone single-component catalysts for enantioselective Buchwald-Hartwig reactions. We have also developed methods for the synthesis of reaction substrates to test the intramolecular asymmetric Buchwald-Hartwig reaction for the preparation of larger and smaller rings, as well as substrates for intermolecular desymmetrization reactions. These studies are currently ongoing.
All of this work has been carried out by students at the undergraduate and Masters level.