57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR1049390-UR10: Enhancing Conversion Efficiency of Dye-Sensitized Solar Cells by Synthesis of Highly Ordered Titania Structures and Judicious Selection of Redox Couples
Feimeng Zhou, PhD, California State University (Los Angeles)
Summary:
In the past grant year, we have (1) successfully coated the TiO2 nanorod array with dye molecules and blocked sites with organosilane to prevent hole/electron recombination and (2) constructed dye-sensitized solar cells (DSSCs) and evaluated the cell performance. We have also begun to synthesize several redox couples with varying redox potentials to optimize the energetics of our photovoltaic cells.
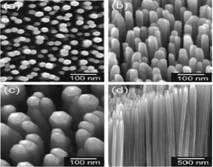
Fig. 1 SEM images of the TiO2 nanorod arrays (Left). Current density J plotted against potential E at the TiO2 nanoarray electrodes (Right) that had been coated with organosilane in: 0 (black), 1 (red), 2 (blue), 3 (cyan), 4 (magenta), and 5 (yellow) cycles of coating (panel a: under the illumination of light equivalent to one sun) and (panel b: in the dark). The acetonitrile solution contained ferrocene/ferrocenium (Fc/Fc+) as the redox couple.
The scanning electron microscopic (SEM) images on the left of Fig. 1 are TiO2 nanoarrays shown in different dimensions and angles. These nanoarrays were synthesized using the method we modified or adopted from literature methods. By coating the rods in the array with organosiline molecules of different thicknesses, we can systematically increase the photocurrents under light illumination (panel a on the right of Figure 1) and in the dark (panel b). The thickness of the silane coating is dependent on the number of coatings, which in turn affect the current density. As shown in Figure 1, the current density and voltage values increase with the number of coatings when the electrode is illuminated under light equal to one sun. However, in the dark, while the voltages increase with the number of coatings, the current density is the highest when only three layers of coatings were applied. This result suggests that when the coating is too thick, the electron transfer between the electrode and the redox species in solution might have been impeded.
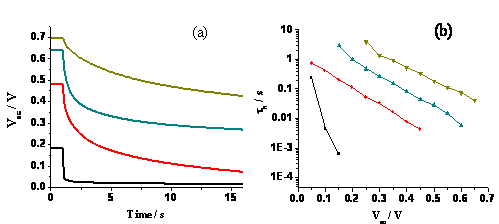
Similarly, we found that a thick organosilane coating also helps retain the open-circuit cell voltage and prolongs the lifetime of the photoelectrons and holes (Figure 2a and 2b, respectively). This is again due to the greater blockage of the surface hole/electron recombination reaction imparted by the thick insulating coating.
Fig. 2 (a) Open-circuit voltage decay and (b) lifetimes of DSSCs using electrodes of TiO2 nanorods coated with organosilane molecules upon 0 (black), 1 (red), 3 (cyan), and 5 (yellow) cycles of coatings. Fc/Fc+ was used as the redox couple in acetonitrile.
Table 1 lists the various cell parameters (short-circuit current density Jsc, open-circuit voltage (Voc), fill factor and efficiency, and compares them to those obtained by Feldt et al. who also used the Fc/Fc+ as the redox couple. As can be seen, the three cycles of organosilane coating (shown in bold and italics) yielded the highest efficiency, which is more than twice as high as that achieved by Feldt et al. (also shown in bold and italics at the bottom of the table). We attributed this enhancement in efficiency to our effective coating as well as the use of arrays of TiO2 nanorods instead of nanoparticles (higher order is expected from the arrays of nanorods).
Table 1. Cell parameters and comparison to representative literature value
Coating cycles | Jsc (mA/cm2) | Voc (Volt) | Fill factor | Efficiency(%) | References |
0 | 0.224 | 0.190 | 0.263 | 0.011 | Our work |
1 | 0.852 | 0.486 | 0.587 | 0.243 | Our work |
2 | 1.559 | 0.573 | 0.658 | 0.588 | Our work |
3 | 2.043 | 0.634 | 0.578 | 0.749 | Our work |
4 | 1.049 | 0.660 | 0.572 | 0.396 | Our work |
5 | 0.660 | 0.690 | 0.634 | 0.289 | Our work |
2 | 1.020 | 0.553 | 0.642 | 0.360 | Feldt et al.1 |
1 Feldt, S. M.; Cappel, B. U.; Erik, M. S.; Anders, H. J. Phys. Chem. C 2010, 114, 10551.
The above results are quite promising and we are in the process of writing a manuscript to report on our findings. We envision that, with the use of a more suitable redox couple (for obtaining better energetics with respect to the band gap of TiO2) and better surface treatments (more complete blockage of the surface electron/hole recombination), the overall efficiency can be further enhanced. Moreover, we have embarked on an endeavor to immobilize quantum dots (QDs) onto the arrays of TiO2 nanorods with the expectation that both the light absorption and tuning of the energetic can be improved.
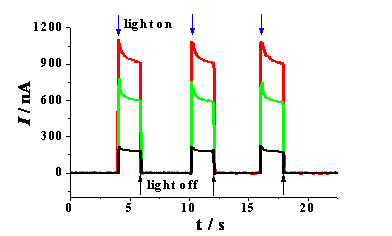
The DSSC project also helped spawn other related projects. For example, immobilization of QDs onto optically transparent electrodes enabled us to use a photoelectrochemical cell for sensitive and selective detection thiol-containing compounds (cf. Figure 3). Attachment of the QDs was accomplished by layer-by-layer deposition of the CdS QDs using positively charged poly(diallyldimethylammonium chloride) as the interlayer molecules.
Figure 3. Photocurrent responses of a (PDDA/CdS QD)-modified electrode in 0.1 M phosphate buffer before (black curve) and after (red curve) being soaked in 10 mM Na2S solution for 10 min. The green curve was acquired after further immersion of the electrode in a 50 nM cysteine solution.
Student developments: Angela Ramos, an undergraduate student supported in the first two years of the grant, has entered our M. S. graduate program and is now a graduate fellow of the NSF-CREST-funded Center for Energy and Sustainability. Estuardo Monterroso, who was also supported by this grant, will be a graduate student in our program in early 2013. In this grant year, Sevak (Sam) Ghazarian and Dong (Jimmy) Kim worked as undergraduate researchers on this project, with the help from Angela and others. The project has excited all these students and motivated them to pursue Ph. D. degrees in chemistry or materials science.