57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR451031-UR4: The Kinetics of Aggregating Dyes
Peter J. Collings, Swarthmore College
Finishing Up the Investigation of IR-806
Understanding the assembly process and liquid crystal properties of IR-806 was part of our previous PRF grant and two remaining tasks needed to be done in order to finish the investigation. First, we applied for and were granted time at the National Synchrotron Light Source (Brookhaven National Laboratory) to perform x-ray diffraction measurements on IR-806. These were successful, showing that the main assembly mechanism is a stacking of molecules, but that the final assemblies that form are more complex and much larger than a single or double stack of molecules. Second, we developed a simple theory with two stages of assembly to model our data on IR-806. At lower concentrations assemblies are formed via an isodesmic process. Above a higher threshold concentration, the assemblies form a more complex and larger assembly. Both of these processes are shown in the model plot below. It is the larger assemblies that orientationally order to form the liquid crystal phase. All of our results for IR-806 are described in a manuscript submitted to the Journal of Physical Chemistry B and now under review.[1]
Investigating the Kinetics of Assembly in Chromonic Liquid Crystal Systems
The main goal of the current grant is to utilize kinetics experiments to understand the assembly and dis-assembly process leading to chromonic liquid crystals. Preliminary kinetics experiments had been performed in the laboratory of Heinrich Roder at the Fox Chase Cancer Center, but the grant allowed us to purchase the necessary instrumentation to do the experiments at Swarthmore College. Our first investigation was to strengthen the finding of a critical assembly concentration using kinetics experiments. We therefore conducted an investigation of Benzopurpurin 4B and IR-806.
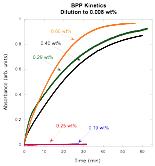
In prior work [2], we studied Benzopurpurin 4B using a variety of techniques. There was no sign of a critical assembly concentration, but the evidence was that large assemblies form, which is something that might occur at a critical concentration. The absorption spectrum of Benzopurpurin 4B does not change a great deal as assemblies form, but we knew the kinetics were quite slow. So we investigated changes to the absorption spectrum immediately after a dilution. To our surprise, it was quite evident that a critical assembly concentration exists at 0.25 wt%. As can be seen in the following plot, above 0.25 wt% there is a clear change in absorbance as the assemblies fall apart due to the dilution, while at 0.25 wt% and below, there is no change in absorbance after the dilution takes place. This process is clearly a second step in the assembly process, since the absorption spectrum changes continuously with changes in concentrations below 0.1 wt%. Thus the assembly process is Benzopurpurin 4B follows the theoretical model we developed for IR-806.
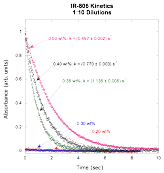
Prompted by the absorption changes in IR-806, we performed kinetic experiments on IR-806 by diluting concentrations in the neighborhood of the suspected critical assembly concentration. As can be seen in the plot, there is clearly a change in the absorption caused by the dilution for concentrations above 0.3 wt%, but not for concentrations equal to 0.3 wt% and below. This matches the finding from absorption measurements, where the spectrum change starts to occur at 0.3 wt%.
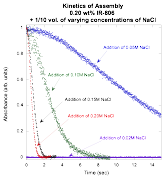
Both absorption changes and preliminary kinetic experiments indicated that assembly of the largest structures in IR-806 can be prompted by the addition of salt to the solution. Therefore, different concentrations of salt solutions were added to an IR-806 sample with a concentration just below the threshold for assembly. The plot below shows the kinetic curves and indicates that addition of a 0.02 M NaCl solution does not cause assembly, whereas addition of a 0.05 M or higher NaCl solution does cause assembly to occur. In addition, the higher the NaCl concentration, the higher the rate constant. As noted in our preliminary work, the kinetic curves follow a stretched exponential function, which is what one would expect for a distribution of assembly sizes.
Pinacyanol Acetate
Assembly in pinacyanol acetate has been reported to have many similarities to what is observed in IR-806.[3] So we synthesized the compound and performed several experiments on it. First, we mapped out its phase diagram for the first time. Second, we studied the changes to its absorption spectrum as a function of concentration. As with IR-806, the absorption spectrum changes dramatically, so we performed a Gaussian decomposition to look more closely at the individual peaks that make up its spectrum. There is evidence for isodesmic assembly at low concentrations, but at higher concentrations one peak continues to increase in intensity while the other peaks decrease in intensity. There does not seem to be evidence for a critical assembly concentration. We plan to perform kinetics experiments to look more carefully for a critical assembly concentration.
[1] Mills, E. A.; Regan, M. H.; Stanic, V.;Collings, P. J. J. Phys. Chem. B (submitted).
[2] McKitterick, C. B.; Erb-Satullo, N. L.; LaRacuente, N. D.; Dickinson, A. J.; Collings, P. J. J. Phys. Chem. B 2010, 114, 1888-1896.
[3] Rodriguez-Abreu, C.; Torres, C. A.; Tiddy, G. J. T. Langmuir 2011, 27, 3067-3073.