57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI350974-DNI3: Synthesis of N-Heterocyclic Carbene-Dithiolate Pincer Ligands and their Transition Metal Complexes: Investigation of Reactivity and Ligand Redox Behavior
Christine M. Thomas, PhD, Brandeis University
Summary of Proposed Work
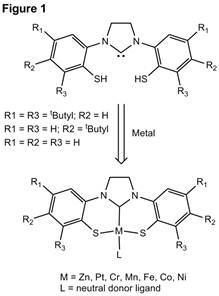 The
proposed project involved the investigation of a series of N-heterocyclic
carbene-containing dithiolate pincer ligands and their coordination chemistry
with a series of transition metals (Figure X). Group 10 metal complexes featuring
the parent ligand (all R groups in Figure 1 = H) had been previously
synthesized by Sellmann and coworkers via a one-pot route, and we had hoped to
expand the coordination chemistry of this ligand variety to a number of different
metals. As detailed below, this proved more challenging than originally
anticipated, but we have started to target a new series of ligand derivatives
with many similar properties to the initial target that may also be able to
support ligand-based redox behavior.
The
proposed project involved the investigation of a series of N-heterocyclic
carbene-containing dithiolate pincer ligands and their coordination chemistry
with a series of transition metals (Figure X). Group 10 metal complexes featuring
the parent ligand (all R groups in Figure 1 = H) had been previously
synthesized by Sellmann and coworkers via a one-pot route, and we had hoped to
expand the coordination chemistry of this ligand variety to a number of different
metals. As detailed below, this proved more challenging than originally
anticipated, but we have started to target a new series of ligand derivatives
with many similar properties to the initial target that may also be able to
support ligand-based redox behavior.
Independent synthesis of NHC-dithiolate ligand
Our first goal was to develop an independent synthesis of the NHC-dithiolate ligand to allow for more thorough and straightforward examination of its coordination chemistry. Typically, imidazolium salts are synthesized via addition of triethylorthoformate to a diamine precursors. However, treatment of the corresponding diamine/dithiol precursor in our case instead leads to the dithiazolium salt, where the carbon atom has inserted between the N and S atoms, rather than between the two N atoms. Based on this, we attempted a number of thiol protection strategies but unfortunately these routes all proved unsuccessful. Different synthetic routes to the ligand, including metal-templated syntheses, nucleophilic aromatic substitution of aryl halides, and the usage of a diimine/dithiol precursor to access the ligand target with an unsaturated backbone were also unsuccessful.
One-pot synthesis
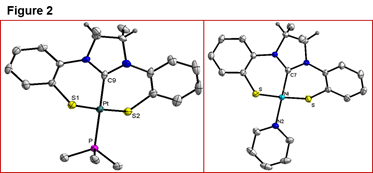 In
the absence of stepwise synthetic methodology, we turned our attention back to
the one-pot route reported by Sellmann (incorporating both the metal and the
NHC carbon in a single step), adding methyl groups to the C4 and C5 positions
of the NHC backbone to improve solubility. This route worked beautifully for
Ni, Pd, and Pt and we were able to synthesize (NHC-S2)2M2
dimers as well as square planar (NHC-S2)M-L monomers (L = PMe3,
pyridine). These complexes were structurally characterized (Figure 2). Unfortunately,
attempts to carry out a one-pot synthesis of transition metal complexes using
other transition metals such as Co, Cu, and Fe were unsuccessful, leading to
intractable mixtures composed of mostly simple metal halide salts.
In
the absence of stepwise synthetic methodology, we turned our attention back to
the one-pot route reported by Sellmann (incorporating both the metal and the
NHC carbon in a single step), adding methyl groups to the C4 and C5 positions
of the NHC backbone to improve solubility. This route worked beautifully for
Ni, Pd, and Pt and we were able to synthesize (NHC-S2)2M2
dimers as well as square planar (NHC-S2)M-L monomers (L = PMe3,
pyridine). These complexes were structurally characterized (Figure 2). Unfortunately,
attempts to carry out a one-pot synthesis of transition metal complexes using
other transition metals such as Co, Cu, and Fe were unsuccessful, leading to
intractable mixtures composed of mostly simple metal halide salts.
Redox activity of NHC-dithiolate ligand
With the Ni NHC-dithiolate complexes in hand, we set out to evaluate the potential redox activity of the ligands. As an important control, we synthesized an analogous complex in which the thiolate donors had been converted to thioethers, allowing us to assess the role that the thiolates played in redox chemistry. Cyclic voltammetry studies of both the NHC-dithiolate and NHC-dithioether Ni complexes showed an oxidation around 0 V (vs Fc). The dithiolate complex did undergo a further oxidation around 0.4 V vs Fc, but a comparison of the two cyclic voltammograms indicates that the oxidation process is metal-based rather than ligand-based (Figure 3). Nonetheless, attempts were made to chemically oxidize the NHC-dithiolate Ni complexes (using Ag+, I2, NO+, and Fc+), but these reactions led to ligand degradation and/or intractable mixtures of products. The NHC-dithioether control compounds could be coordinated to Fe and Co, but the analogous dithiolate complexes could not be synthesized for comparison.
New Ligand Target
Since the initially proposed ligand series was more synthetically challenging than originally anticipated, a new interested ligand target has been identified. While a number of aryl-based bidentate redox active ligands have been investigated with O, S, and N donors, there are currently no examples of similar P-based ligands: [NP]2-. Thus, we have begun to investigate aryl-linked phosphide/amido ligands and their coordination chemistry. Preliminary computational data on PtII complexes of the [NP]2- ligand suggest that these ligands will also display redox active behavior. As shown in Figure 4, the HOMO of the Pt complex [NP]Pt(dmpe) (dmpe = bis(dimethylphosphino)ethane) is ligand-based, with most electron density on the amido nitrogen donor and the aryl ring linking the P and N atoms. A one electron oxidation of this compound is predicted to occur at the ligand (to form [NP]-∙) rather than the metal (for form PtIII), as illustrated by the calculated SOMO of the cation [NP]Pt(dmpe)+. The [NP]2- ligand has been synthesized and coordinated to Pt, forming the phosphide-bridged Pt dimer {[NP]Pt(PMe3)}2, whose structure is shown below in Figure 4. Future studies will focus on examining the redox chemistry of this Pt complex, as well as the coordination chemistry of the [NP]2- ligand with a series of transition metals.
Impact of the Research
While the initial goals of this research project were not attained, the new research aims promise to lend insight into the design of redox active ligands. Specifically, we hope to ascertain whether phosphorus-based donors can support the same type of ligand-based redox behavior as nitrogen-based donors. This fundamental information will provide insight into the development of catalysts for multielectron redox processes such as those involved in the activation of s bonds in alternative fuels.
The research has, thus far, impacted by career by allowing me to explore new and interesting fundamental research directions. We were able to quickly establish a research area that was not worth pursuing and move on to a new, but related, direction that so far seems more promising. This sort of exploratory freedom is extremely important for a new PI as they establish their niche in chemical research.
The postdoctoral fellow supported by this grant and the graduate student working closely with him on the project have learned a lot through the successes and failures of this project. They have a lot about synthetic inorganic chemistry, including characterization methods such as NMR spectroscopy and X-ray crystallography, and about chemical redox processes and how they can be probed using cyclic voltammetry, spectroscopic methods, and key control experiments. Most importantly, they learned a valuable career lesson about research: ideas that look reasonable on paper are not always as straightforward and synthetically accessible as they seem.












