57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR550594-UR5: Fundamental Studies of Diffusion and Retention on Porous Polymer Monolith Stationary Phases Used in Capillary Electrochromatography
Michelle Marie Bushey, Trinity University
We are investigating organic porous polymer monoliths (PPMs) that are used in capillary electrochromatography (CEC) applications. More specifically, we are investigating fundamental properties of lauryl acrylate polymers to better understand the nature of the analyte, stationary phase, and mobile phase interactions. This is approached by measuring the diffusion coefficients of a variety of analytes with different retention factors, under different conditions, to determine the relationship between diffusion and retention as well as to examine the thermodynamics of the retention and diffusion processes. In traditional chromatography systems, utilizing familiar stationary phases comprised of C18 bonded to silica surfaces, the relationship between diffusion and retention has typically been described with the Knox equation given below in eq 1 where Deff is the effective diffusion coefficient of the analyte in the presence of the stationary phase, gm is the column obstruction factor, Dm is the diffusion coefficient of the analyte in the mobile phase, k is the retention factor, and gsDs is the kinetic parameter, comprised of the obstruction within the stationary phase and diffusion within the stationary phase.
Deff = (gmDm + kgsDs)/(1+k) (1) (1+k) Deff /Dm = gm + kgsDs/Dm (2)
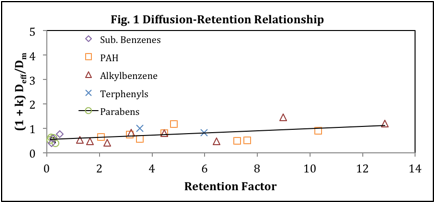
Eq 2 is simply a rearrangement of eq 1 and shows that a plot of the left hand side versus k should result in a straight line where the intercept will yield gm and the slope will yield the kinetic parameter. However, all investigations using C18 bonded to silica supports have resulted in plots that show negative deviations from linearity. There are two explanations for this behavior. One is that the kinetic parameter is not a constant but rather a function of retention. The second is that the Knox theory is in fact incorrect and does not account for the two types of diffusion that occur within the stationary phase. Our results for a lauryl acrylate PPM stationary phase, utilizing a 75:25 ACN:5 mM Tris mobile phase, yields dramatically different results. Ideal Knox behavior is observed. A straight line is achieved, and the kinetic parameter is greatly reduced from those typically seen in silica based stationary phase studies. We have attributed these differences to two properties of the PPM that set it apart from silica based stationary phases. The adherence to linearity is attributed to a relative lack of mesopores in the polymer monolith which in turn results in far less stagnant mobile phase zones. Furthermore the greatly reduced kinetic parameter is attributed to the structure of the polymer surface which is markedly different from the surface of a C18 bonded phase in terms of the likely presentation of the carbon chains but also in terms of the distribution of charged moieties. Our results, obtained for a set of 25 analytes covering a retention range of less than one to over 12 is shown in Fig. 1. These results have just been submitted for peer reviewed publication.
In another facet of our study, we are examining the thermodynamics of retention on the same phase. Analyte retention is measured as a function of temperature, covering a temperature range of 25-60 degrees. From the plots of ln k versus the inverse of the temperature, van't Hoff plots, the enthalpy and entropy of retention can be obtained. We are currently conducting experiments to determine the phase ratio of these polymer preparations. When completed, we will be able to calculate the entropy of retention. We expect to complete this data analysis very soon and a manuscript draft is in preparation.
This grant has supported students in the summers of 2011 and 2012. To date nine individual students have been involved with this research. A total of five students have worked on this project in each summer and three of these were fully supported by this grant. The nature of the project requires a substantial amount of careful, time consuming data collection which in turn requires a sizeable research team. We would not now be preparing to publish two distinct papers as a result of this research if fewer students had participated. Two students worked on this research in both the summers of 2011 and 2012 and the intervening academic year. One of these is currently applying to graduate schools to complete PhD studies in analytical chemistry while spending his senior year abroad. All students who participated in this research in the summer of 2011 continued for all or part of the 2011-2012 academic year. Of the five students who participated in the summer of 2012, three are currently still participating. Of those who participated in the summer of 2011, two presented posters at the 243rd American Chemical Society meeting in March, held in San Diego. One of the others gave a talk at Pittcon 2012 in March in Orlando, and the other two copresented a poster at Pittcon 2012. Of the five who participated in the summer of 2012, two have oral talk abstracts submitted for Pittcon 2013 and two have poster abstracts submitted. Two postdoctoral associates, funded by other sources, have also participated in research related to this grant. They have also been partially responsible for supervising these undergraduate researchers. The first postdoc has recently completed one year in this laboratory and has now begun a tenure track appointment at a predominantly undergraduate institution. The second postdoc is at the beginning of her appointment in this laboratory. Funding from the ACS PRF has revitalized my undergraduate research program.