57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI1050201-DNI10: Fundamental Studies of Lithiation/Delithiation Processes in V2O5 and MxV2O5 Nanostructures
Sarbajit Banerjee, PhD, State University of New York at Buffalo
1. Overview
The discovery of novel electrode materials for Li-ion batteries remains primarily guided by empirical considerations. Robust design principles for identification of materials, redox centers, and appropriate dimensionalities remain to be identified. Our ACS PRF-funded project has been primarily directed at gaining fundamental mechanistic understanding of lithiation/delithiation processes in V2O5 and metal vanadate nanostructures as model systems for cathode materials used in Li-ion batteries. Towards this end, we have pursued a combination of dimensionality and morphology controlled syntheses and ensemble- and single-nanowire studies of lithiation/delithiation processes. In our previous report, we presented a detailed X-ray absorption fine structure (XAFS) spectroscopy and density functional theory investigation of the lithiation of the multi-electron redox material Ag2VO2PO4 and mapped out the orbital-specific progression of the reduction process. Over the course of the last reporting period, our research has focused on the synthesis of open-framework CaxV2O5 nanomaterials and the study of lithiation processes in individual V2O5 nanowires.
2. Findings
2.1. Synthesis and Reversible δ→β Phase Transformations of CaxV2O5: Nanoscale materials often exhibit phase stabilities and phase transitions that are very distinct from their bulk counterparts. In designing a high-surface-area open framework double-layered CaxV2O5 phase, we have encountered the remarkable interconversion of CaxV2O5 between double-layered δ and tunnel-like β phases. The hydrothermal reaction of Ca(NO3)2 or Ca(CH3COO)2 with V2O5 yields single-crystalline nanowires of δ-CaxV2O5 that extend hundreds of microns in length with a unique hydrated coordination environment for intercalated Ca2+ ions. Annealing these nanowires at 500°C for 3 days leads to dehydration of the nanowires to an entirely different crystal structure: the tunnel-like β-phase without a substantial change in the nanowire dimensions or morphology. Hydrothermal treatment of β-CaxV2O5 causes reversion to the hydrated δ-phase. Such reversible interconversion between the two polymorphs has not been observed in the bulk, and arises from the ease of water intercalation and facile accommodation of slip processes possible in nanostructures. Figure 1 shows the structural progression and changes in the local Ca coordination environment during the phase transition. Lithiation processes in δ- and β-MxV2O5 nanostructures have been independently examined thus far since both structures allow for substantial scope for accommodation of Li-ions through ion-exchange. Our results indicate the first feasible connection between the separate phase diagrams at the nanoscale level. This work was published in Inorganic Chemistry.
In very recent work, we have devised independent hydrothermal routes for preparation of δ and β phases of AgxV2O5 by simply altering the ratio of the Ag-precursor in a reaction of silver nitrate with V2O5. Access to both double-layered and tunnel phases of AgxV2O5 provides simpler model systems compared to the previously examined Ag2VO2PO4 (reported by us last year) and will permit correlation of structure-type to lithiation/delithiation behavior and kinetics.
2.2. Synthesis of V2O5
Nanostructures and Single-Nanowire Measurements: Consistent with our theme of
combining synthesis and ensemble characterization with single-nanowire studies,
we have adopted two different synthetic routes to prepare single-crystalline
orthorhombic V2O5 nanostructures: (a) chemical vapor
transport of V2O5 on Si/SiO2 substrates with
deposited Ni- or Co catalysts, and (b) hydrothermal preparation of V3O7
nanostructures followed by oxidation in air at 240°C to obtain
single-crystalline V2O5 nanostructures. The nanowires
have been characterized by X-ray diffraction, Raman spectroscopy, and
high-resolution transmission electron microscopy. Chemical vapor transport
yields phase pure nanowires that are 60-80 nm wide and 1-5 μm long with
tapered ends, whereas hydrothermal synthesis yields 100-200 nm diameter wires
that extend several hundreds of micrometers in 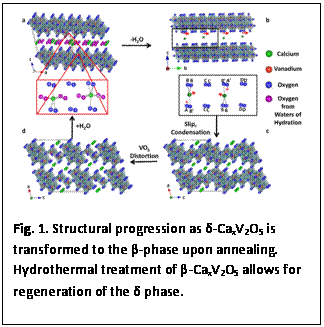 d-CaxV2O5 is transformed to the β-phase upon annealing. Hydrothermal treatment of β-CaxV2O5 allows for regeneration of the δ phase.
">length.
d-CaxV2O5 is transformed to the β-phase upon annealing. Hydrothermal treatment of β-CaxV2O5 allows for regeneration of the δ phase.
">length.
|
|
For ensemble samples, lithiation has been performed by immersion of the nanowires within a 2.5 M n-butyllithium solution in hexane within an argon-filled glovebag for time periods ranging from 1-60 min. For single-nanowire experiments, the nanowires grown by vapor transport are first "harvested" from the growth substrate by ultrasonication in 2-propanol and dispersed onto clean Si/SiO2 (300 nm) surfaces. Individual nanowires are then located using scanning electron microscopy and metal electrodes are patterned using a focused ion beam. Figure 2 shows an individual V2O5 nanowire that has been thus isolated. The substrate is then immersed in a 2.5 M n-butyllithium solution for 1-60 min and Raman spectra are acquired for the single nanowire.
The bulk samples have also been characterized by X-ray absorption fine structure spectroscopy. The spectral features in the near-edge X-ray absorption fine structure region of the V K-edge are particularly sensitive to the nominal vanadium oxidation state, the extent of distortion of the coordination geometry from a perfectly octahedron, the coordination number, and bond distances between the central vanadium atom and the nearest-neighbor oxygen ligands. The pre-edge feature at the V K-edge can be attributed to transitions from V 1s core states to V 3d orbitals that have acquired substantial ungerade p character as a result of hybridization with V 2p and O 2p orbitals. Upon lithitation a pronounced shift of the pre-edge feature to lower energies is noted, which can be attributed to the sequential reduction of V5+. A 2D plot of peak position and intensity allows for evaluation of the change in the local vanadium coordination environment as a function of the extent of lithiation. Ongoing experiments are focused on rigorously excluding air and moisture from the XAFS cell to enable quantitative determination of nominal vanadium oxidation state and local coordination geometry during each phase of the lithiation process.