57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI151259-UNI1: The Design, Synthesis, and Characterization of 'Smart' Donor-Acceptor Biaryls
Bartholomew James Dahl, PhD, University of Wisconsin (Eau Claire)
Research Area 1: Synthesis and Characterization of Donor-Acceptor Biaryl Lactone pH-Driven Molecular Switches
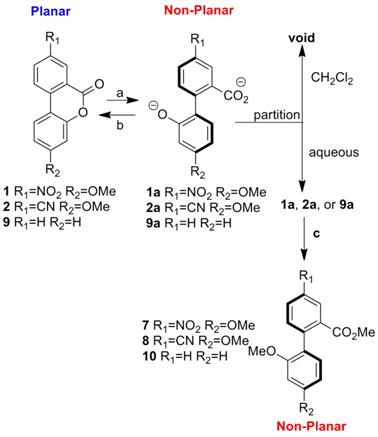
The ACS-PRF was the major funding source for this project. pH-driven biaryl lactone dihedral angle switches 1 and 2 have been synthesized in multi-gram quantities following the steps shown below. Two undergraduate student researchers, Asia Riel and Erick Carlson (both supported internally), performed all of the synthetic work. Both students presented their work at spring 2012 ACS conference in San Diego (supported by PRF funds). We have also submitted a paper to Tetrahedron Letters describing this work and it was accepted for publication on September 6, 2012. One of the research students (Asia) is still at UW-Eau Claire and the other (Erick) is now in graduate school for medicinal chemistry at the University of Minnesota.
Both switches were determined to rapidly switch between the ring-opened (non-planar) and ring-closed (planar) states upon addition of acidic or basic stimuli. Compounds 1/2 ring open to 1a/2a in the presence of base and lactonize in the presence of acid. The figure below shows that process. Compounds 1a/2a were trapped as compounds 7/8 to confirm the identity of the ring-opened structures since they are water soluble and difficult to isolate.
These compounds are not only switches of dihedral angle but also of intramolecular charge transfer (ICT) between the donor and acceptor groups. Since the conjugation is attenuated in the ring opened state (1a and 2a), the charge transfer process is also attenuated. This process was readily probed by UV-Vis and fluorescence spectroscopy. The absorbance of compounds 1a and 2a are both greatly diminished relative to 1 and 2. We have also determined that the luminescence of 1 and 2 can be immediately diminished in the presence of base (switching to 1a and 2a) and then reformed after addition of acid.
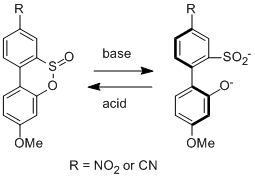
The ACS-PRF was the major funding source for this project. The sultines below should also act as pH driven molecular switches that should behave analogously to the biaryl lactone switches. We are currently working on making both of them. One undergraduate student researcher, Joel Patrow (supported 100% by PRF), started working on the project in the summer of 2012 has been making progress. We have had much success performing many of the reactions but a Pd-catalyzed thiolation has been problematic. To remedy this situation a slightly different route has been proposed, making use of an SNAr reaction, and progress is currently being made.
Research Area 3: Synthesis of Terphenyl Dilactone Switches
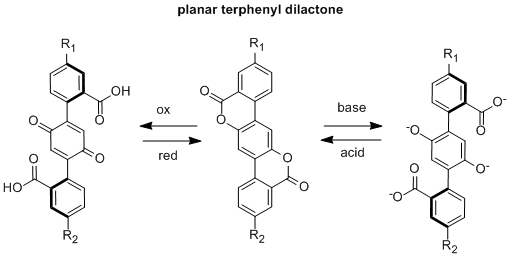
The ACS-PRF was the major funding source for this project. We are currently working on the synthesis of terphenyl dilactones which should act as both redox and pH switches. This work began in summer 2012. Two student researchers, Asia Riel (supported by a McNair Scholarship) and Zach Ahola (supported 100% by PRF), were working on this project.
Our initial research goal was to synthesize unsymmetrical terphenyl dilactones in which R1 was a donor group and R2 was an acceptor group. These types of compounds should be pH and redox-driven switches of geometry and ICT. We found this synthetic route to be quite challenging despite attempting numerous synthetic strategies. However, symmetric terphenyl dilactones, where R1 = R2 should still act as molecular redox or pH switches, although they will not disrupt intramolecular charge transfer upon switching. We decided to focus on the synthesis of these systems near the end of summer 2012 and have been making good progress.
The ACS-PRF funds were used to partially supplement efforts on this project. This project is directly related to research described in the PRF proposal. Currently a manuscript describing this project has been accepted for publication in Tetrahedron Letters (accepted September 14, 2012). No students have been supported on this project with PRF funds (only partial supplies and partial faculty stipend). However, given the promise of this work and the positive reception the paper was given by reviewers, much more focus will go toward this project.
The ACS-PRF funds were used to partially supplement efforts on this project. This project is directly related to research described in the PRF proposal. One student, Nick Sortedahl, was supported (100% by PRF) on this project. Progress toward the synthesis of the compound below is currently being made.
The support from the ACS-PRF has been instrumental in getting these great research results. In my first two years at the University of Wisconsin-Eau Claire (UWEC) I have mentored eight students in research and many of them have been supported by the PRF. The students would have otherwise had a much smaller chance of getting involved in research. Now they have been introduced to the research process and also trained in practical advanced synthetic techniques and characterization. All of my summer research students get training in searching the literature, making presentations, and writing up professional experimental results. Opportunities were also available for two students to attend the spring national ACS conference in 2012 and I plan to take three students with me for the spring meeting in 2013. Other students have presented posters on the UWEC campus for our Student Research Day. These opportunities have excited the students about doing research and most of my research students now plan to attend graduate school. Their performing research in my group will help better prepare them for what they will encounter in graduate school. Without the funding from the PRF, none of this would be possible. Additionally, the PRF has provided seed money for me to get my own research established at UWEC. Thus far, funding from the PRF has resulted in several conference presentations and two published articles in my first year of funding.