57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI550443-DNI5: Adaptable and Smart Amphiphilic Systems in Enhanced Oil Recovery
Mustafa Akbulut, PhD, Texas A&M University
NARRATIVE REPORT
Introduction
Typical EOR techniques aim to achieve one or more of the following: (i) decrease oil viscosity, facilitating the movement of oil within porous oil-bearing rock; (ii) increase the viscosity of water to more effectively sweep oil out from the deposit; and (iii) reduce capillary forces or interfacial tension between oil and the oil-bearing stratum. This report describes the use of novel 'adaptable', and 'smart' amphiphilic systems not only to increase sweeping efficiency but also to decrease to reduce oil-water interfacial tension in an adjustable manner.
Results
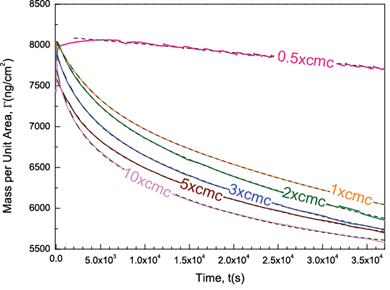
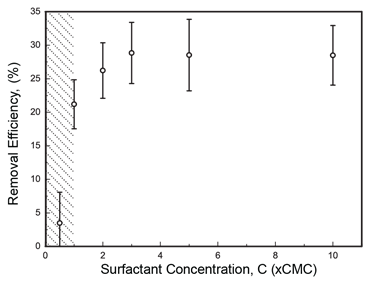
In the last year, we demonstrated that the quartz crystal microbalance with dissipation (QCM-D) can be used to investigate the nanoscale dynamics of oil recovery using surfactant floods. Our further QCM-D studies revealed that for an asphalt film that is exposed to a surfactant flood (polyoxyethylene (n=9) nonylphenylether), the mass of asphalt film remaining on the SiO2 surface as a function of time of surfactant exposure at various surfactant concentrations (Fig. 1). The analysis of this figure revealed the followings: First, the rate of asphalt removal increased with increasing concentration of surfactants. Second, when the surfactant concentration is below critical micelle concentration (CMC, 0.07 g/L), the removal dynamics of asphalt followed a different trend compared to the dynamics obtained at concentrations above CMC. Third, for all surfactant concentrations, desorption data could be well described by two term exponential decay fits, suggesting that two different first-order processes are responsible for the overall removal of asphalt. Fourth, at a 10-hr of surfactant exposure, the asphalt removal efficiency, (min-m10hr)/min, ranged from 21% to 29% depending on the concentration of the surfactant flood (Fig. 2). Below CMC, the fast process disappeared, leading to an inefficient removal.
Figure 1. The mass of asphalt film remaining on the SiO2 surface as a function of time of surfactant exposure at various concentrations and two-term exponential fits for the mass vs. time data (shown with dashed lines).
Figure 2. Asphalt removal efficiency as a function of surfactant concentration upon 10 hr of surfactant flooding.
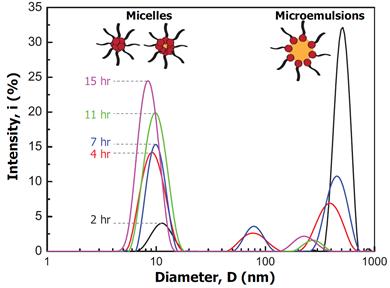
To determine the size characteristics of colloids in the effluent, we used the dynamic light scattering technique. Figure 3 displays the particles size analysis of the effluent that is formed by flowing a 5xCMC concentration of surfactant over the asphalt film as function of time. For all times, the effluent contained micelles that are several nanometers and microemulsions that are a few to several hundred nanometers. The empty (unloaded) micelles were measured to be 2-4 nm in size, suggesting that the micelles shown in Figure 3 are loaded with a trace amount of oil. In addition, the relative ratios of loaded micelles to microemulsions increased with increasing time of flow. Considering the overall removal process has one fast and one slow component, these trends suggest that the fast process is related to the formation of larger microemulsions while the slow process is related to the formation of micelles.
Figure 3. The particle size distribution of the effluent stream, which formed upon exposing asphalt films to a flow of surfactant solution, as a function of time. At CMC, empty (unloaded) micelles had a mean size ranging from 2-4 nm in the absence of oil.
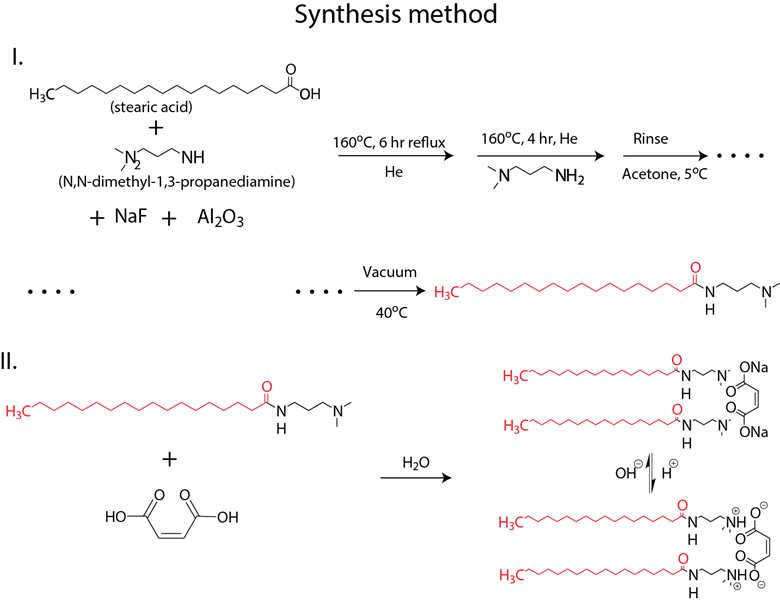
In addition, we synthesized a new amphiphilic molecule, solution of which has adjustable viscosity with respect to pH. The synthesis method used is described in scheme 1. In summary, stearic acid; N,N-dimethly-1,3-propanediamine; NaF; and alumina are refluxed under He at 160° C for 10 hr. Then, the intermediate product is rinsed with acetone and vacuum-dried to obtain amine functionalized stearic acid, which is further reacted with 2-Butenedioic acid in aqueous media. The final product was a gemini amphiphile that can be protonated and deprotonated in the presence of acids and bases.
Scheme 1. Synthetic pathway for pH-switchable long-chain gemini amphiphiles.
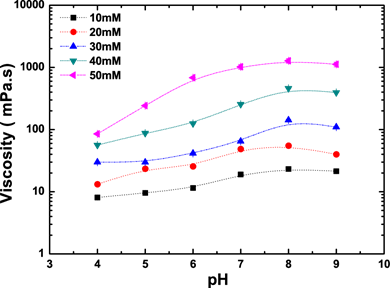
As can be seen from Figure 4, the viscosity of synthesized surfactants strongly depended upon the pH. For instance, at a concentration of 50 mM, the viscosity at pH 4 was 13 times less than the viscosity at pH 9. Presumably, the amphiphile self-assembles into wormlike micelles in the presence of OH- groups , thereby increasing the viscosity of the solution through network formation.
Figure 4. The influence of pH on the viscosity of amphiphilic molecules synthesized.
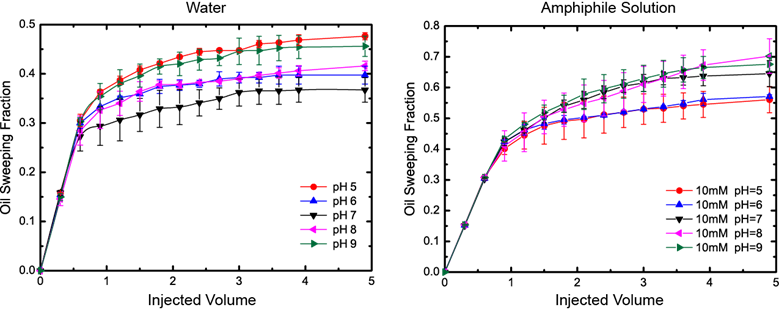
Finally, we investigated the oil sweeping efficiency of the developed amphiphiles using sand columns filled with oil. The expectation was the sweeping (displacement) efficiency of amphiphile solution would be larger at higher viscosities i.e. at elevated pHs. This expectation was experimentally confirmed (Fig. 5): when the developed amphiphile at pH 8-9 and 10 mM concentration is used as displacing media, there was 40-50% increase in the displacement efficiency compared to water at the same pH.
Figure 5. The oil sweeping efficiency of water and amphiphile solutions at various pHs as a function of injected liquid volume (in terms of multiples of the oil volume in the sand column).
Overview and Perspective
In essence, EOR techniques based on abovementioned amphiphiles have the potential to add significantly to the amount and efficiency of oil that can be extracted from mature fields. The two key benefits of the developed amphiphiles are: (i) each oil reservoir generally has different characteristics in terms of its permeability, porosity, crude-oil type, temperature, and water composition. Thus, each requires a different level of tuning in viscosity and interfacial tension. The use of adaptable amphiphiles can alleviate the need to synthesize a different surfactant system for each oil reservoir; and (ii) one way to minimize the energy cost of pumping down flooding water for sweeping oil away is to control the viscosity of the water. Since it is easier to pump down less viscous water, it is desirable to begin EOR with a low viscosity, increase viscosity when sweeping is needed, and decrease viscosity after the sweeping has been carried out. Using abovementioned amphiphiles, such control over the viscosity of water can be achieved by simply adding small amounts of acid and base to the amphiphile solution.