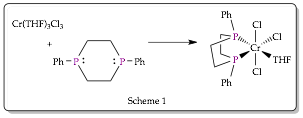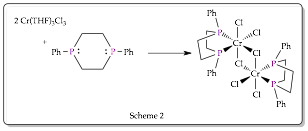57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR350596-UR3: Multidentate Cyclic Phosphines: Synthesis of the Novel 1,4,7-Triphosphacyclone and Linear Alpha Olefin Catalysis Studies Using 1,4-Diphosphacyclohexane
Monte L. Helm, Fort Lewis College
During the grant period for this report we successfully synthesized monomeric and bridged Cr-1,4-diphosphacyclohexane (6P2) metal complexes as shown in Schemes 1 and 2.
The paramagnetic compounds were characterized through elemental analysis. Selective catalytic linear alpha olefin production was pursued using a Parr reactor, standard amounts of catalytic compound and constant ethylene pressure (Scheme 3).
The reaction products were analyzed using GC and linear alpha olefin production was confirmed. The reaction mixtures showed formation of 1-hexene and 1-octene, however, the relative amounts were not quantified at this stage.
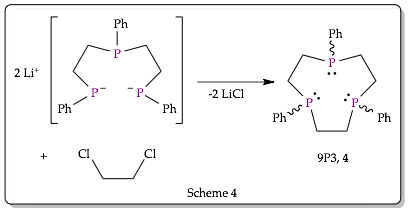
During the grant period the optimization of the synthesis of the 1,4,7-triphosphacyclononane (9P3) ligand was also pursued. The reaction of bis(2-phenylphosphidoethyl)-phenylphosphine with 1,2-dichloroethane results in a ring closing reaction to form the 9P3 ligand (Scheme 4).
Under dilute, high-temperature conditions, the 9P3 ring was found to form preferentially. The presence of the 9P3 ring in the reaction mixture has been confirmed through P-31 NMR and forms in as much as 80% yield based on spectral integration. Optimizing the 9P3 synthesis to use less solvent and lower temperatures (without a loss of yield) was the first major goal of this work and we were able to pursue one of our proposed strategies during this grant period: varying reaction conditions. We systematically explored the effect of different solvents, temperatures and reactant concentrations on the yield and purity of 9P3 synthetic process and found the reaction process was optimized using refluxing toluene, rather than THF as we previously reported. This optimization resulted in a reduction of the overall volume of solvent that is required for efficient formation of the 9P3 ligand.