57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR150612-UR1: New Synthetic Methodology to Prepare Imine Derivatives in Single, Predictable Geometric Configurations
Debra D. Dolliver, Southeastern Louisiana University
The overarching aims of this grant were to devise methodology by which imidoyl halides could be successfully used in synthetic schemes to efficiently synthesize single geometric isomers of oxime ethers. The following report indicates the progress that has been made toward the goals established in this grant.
Progress toward Goal A: Establish a robust synthetic protocol to reliably synthesize single E or Z isomers of oxime ethers using a variety of imidoyl iodides and trifluoroborate salts
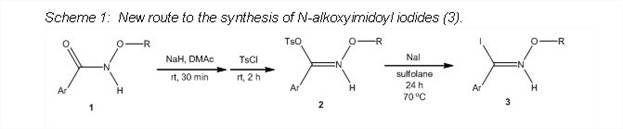
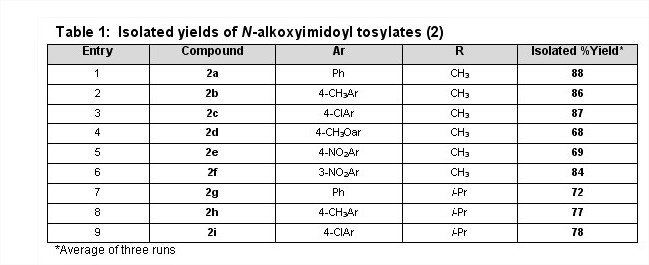
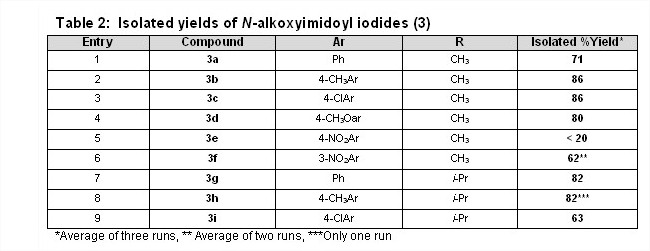
To perform the palladium-catalyzed coupling of N-alkoxyimidoyl iodides, the PI's undergraduates have undertaken the development of an alternate route to the iodo-compounds 3, the advantages of which include shorter reaction times, reagents that are easier to handle and greater ease of purification. To date the isolated yields have been good to excellent for both the N-alkoxytosylates 2 and the N-alkoxyimidoyl iodides 3 with the exception of the synthesis of compound 3e (entry 5, Table 2).
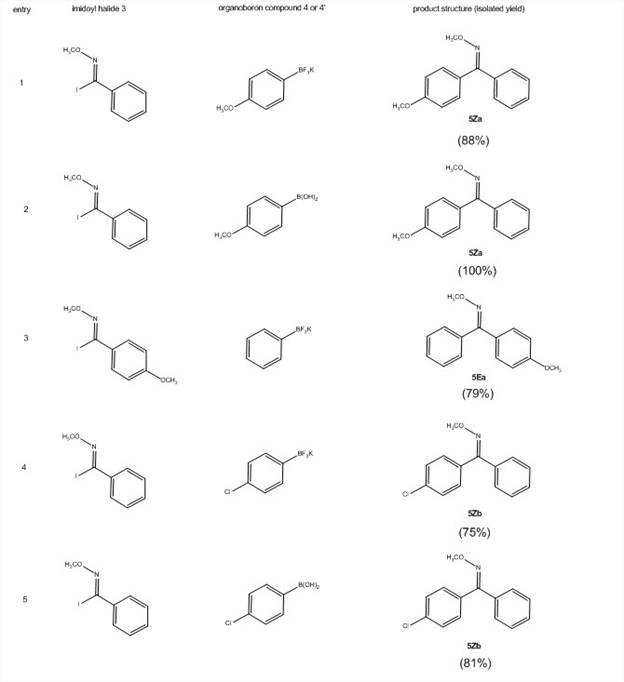
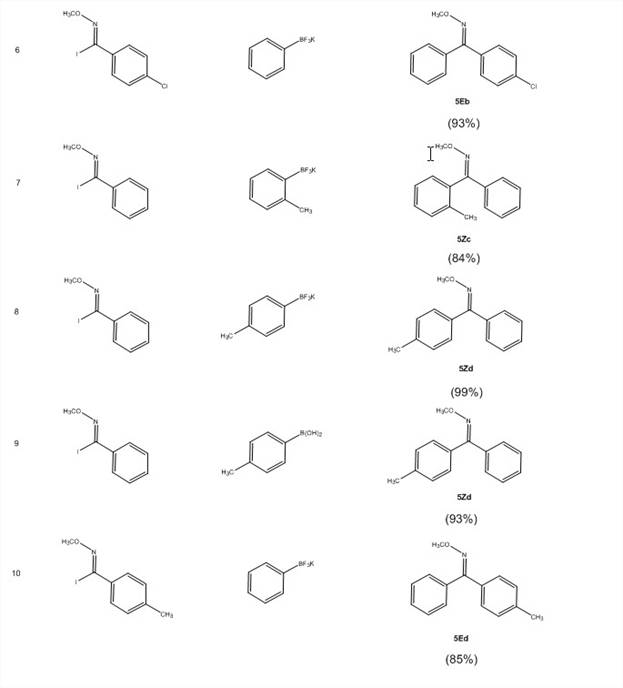
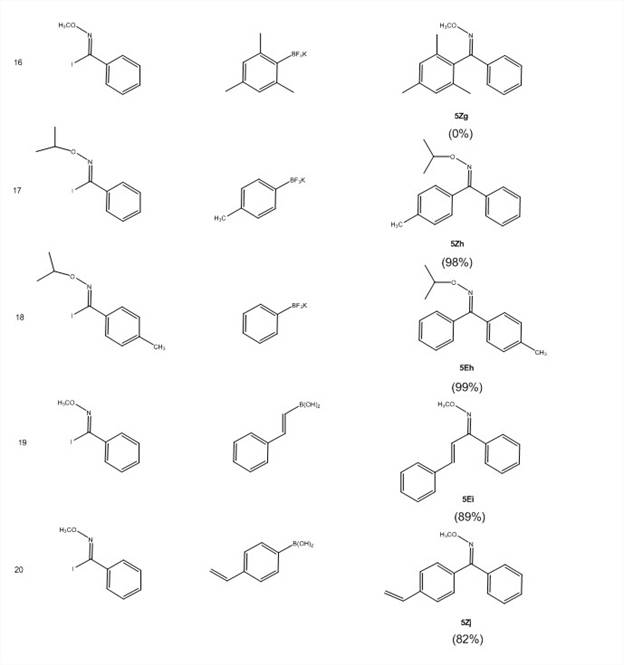
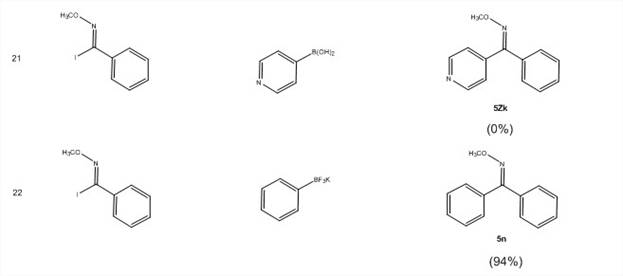
With these imidoyl iodides in hand, the PI's lab has screened for optimal reaction conditions for the Suzuki coupling reactions shown in Scheme 2. They have screened ligands [PPh3, P(t-Bu)3HBF4, dppf, SPhos, no ligand], bases [Cs2CO3, K2CO3, KOH, KF], temperature and concentration. The optimized conditions (Scheme 2) now give excellent yields (Table 3). This reaction efficiently undergoes cross-coupling with low catalyst loading (1%) with electron-donating, electron-withdrawing, and bulky groups on the ring of both the imidoyl iodide 3 with either the boronic acid 4 of the trifluoroborate salt 4′.
Table 3: Isolated yields for Suzuki coupling reactions of N-alkoxyimidoyl halides
The NMR data indicates that only one isomer is formed in this reaction and that the isomers are different depending on whether the ring substituent is on 3 or 4. E/Z geometry is currently being determined by X-ray crystallography on product compounds of 5 that are crystalline. It is anticipated that the work performed under this goal will result in a manuscript submission within the second year of the grant.
Progress toward Goal B: Investigate the above coupling technique with N-alkoxyimidoyl pseudohalides
The Suzuki coupling technique was tried with N-alkoxytosylates 2 using varying conditions. To date, these reactions have failed to give the desired product even under forcing conditions. The Suzuki reaction was also tried with N-alkoxyimidoyl bromide [PhC(Br)=NOCH3] in the place of the iodide compound 3 under the conditions given in Scheme 2. This only resulted in a 43% isolated yield.
Progress toward Goal C: Investigate the coupling technique with other N-substituted imines
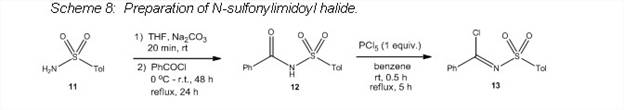
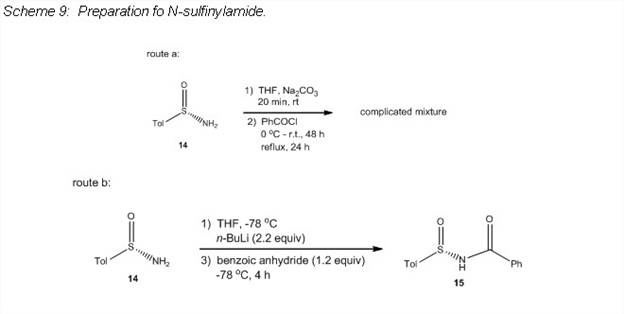
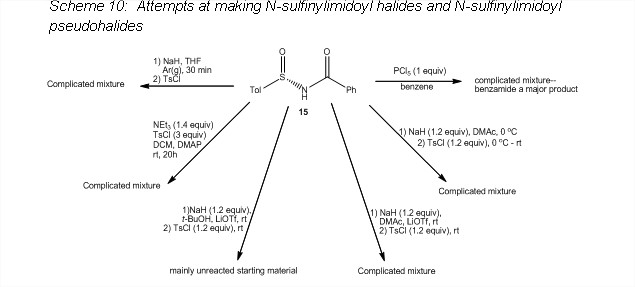
The N-sulfonylimidoyl chloride 13 was synthesized in the PI's lab in yields similar to those reported in the literature (Scheme 8),5 and the reaction served as a template for making the N-sulfinylimidoyl halide. Attempts at making the N-sulfinylamide 15 by the same method as that used to make 12 resulted in a complicated mixture (route a, Scheme 9). However, an alternate route was found which formed 15 in good yield (route b, Scheme 9).6 Unfortunately, reaction of 15 with PCl5 only resulted in a complicated mixture (Scheme 10) and no convincing evidence for formation of the desired product was found.
The PI's group then explored several different routes to form the N-sulfinylimidoyl tosylate or mesylate (Scheme 10). None of these reactions resulted in the desired product, and it appeared that reaction was occurring at the sulfinyl group.
The PI's group also explored a route to making N-sulfenylimidoyl chlorides 19 in the hopes that these compounds could eventually be asymmetrically oxidized to the asymmetric sulfinylimidoyl halide. Compound 18 was successfully synthesized following literature procedures7,8, but the PI's group has been unsuccessful in synthesizing 19 the subsequent step. This moiety has not been reported in the literature.
The PI has recently found evidence in the literature that the N-sulfinimines [ArC(Ar')=NSOR] are not geometrically stable and readily undergo E/Z isomerization, even at low temperatures.9,10 Because of this finding, these compounds are less attractive as candidates for undergoing coupling reactions to form compounds of single E/Z geometry.
In the second year of the grant the PI plans to explore the synthesis of asymmetric hydrazonyl halides and explore their participation in the Suzuki coupling reactions introduced above under Goal A.