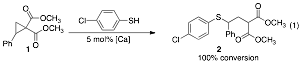57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI151029-UNI1: Calcium Catalyzed Homologous Conjugate Addition Reactions
Kristine Nolin, PhD, University of Richmond
Our group is developing catalytic homologous conjugate addition (HCA) reactions, additions to electron-deficient cyclopropanes, utilizing calcium(II) complexes to facilitate the reactions. While many methods exist for catalytic 1,4-additions of nucleophiles to electron deficient conjugated ¹-systems, the homologous reaction has yet to be fully realized. HCA reactions are usually performed under forcing conditions using stoichiometric or super-stoichiometric amounts of a Brznsted base and/or Lewis acid. Unlike Lewis acid metals commonly used in synthetic methodologies, calcium complexes are relatively inexpensive and many are environmentally benign. The large radii and electropositive nature of calcium leads to a similarity in coordination behavior and observed reactivity to lanthanide metals.
Initial Results
During the initial stages of this investigation, the addition of unactivated thiols to doubly activated cyclopropanes, those containing two electron-withdrawing groups, is being explored. After a short screening, we were pleased to identify a commercially available calcium(II) complex that catalyzed the addition of 4-chlorothiophenol to cyclopropane 1 leading to the quantitative conversion of 1 to g-sulfanyl malonate 2 (eq 1). It should be noted that no exogenous base was required and only one regioisomer of the product was observed.
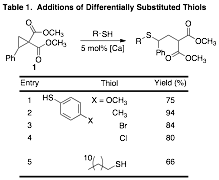
After reaction optimization, we began to test the generality of the reactions to different thiols. Initial results show that electron-rich and electron-deficient thiophenol derivatives readily add to 1 providing the corresponding g-sulfanyl malonates in good to excellent yields (Table 1, entries 1-4). As our first example of alkyl thiol reactivity, dodecanethiol was added to 1 in good yield (Entry 5, 66%). We are still working on optimizing of our purification methods, which should lead to increased yields as well as examining an increased breadth of thiols.
Students have begun to synthesize and react differentially substituted, doubly activated cyclopropanes. Our first targets have been cyclopropanes with electron-rich or electron-deficient aryl substituents. The synthesis of the cyclopropanes has been challenging with regards purification. However, we have purified and reacted three additional cyclopropanes under the standard reaction conditions and were pleased to find that the addition reactions proceeded in very good yield (Table 2, entries 1-3). Future reactions will include examination of alkyl-substituted cyclopropanes.
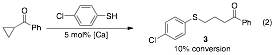
In addition to doubly activated cyclopropanes, preliminary exploration of singly activated cyclopropanes has also begun. The commercially available cyclopropyl phenyl ketone was reacted under the standard reaction conditions. We anticipated that this would be a challenging substrate; therefore, we were excited to see that it was reactive, albeit modestly. The first reactions proceeded with 10% conversion of the cyclopropane to g-sulfanyl malonate 3 in the presence of 5 mol% of the calcium complex. This is proof of principle and the foundation of future reaction development.
Impact
During the first year of ACS-PRF support, one research student was supported during summer research (two next year). The student made tremendous progress on this project and was able to gain experience in reaction optimization, synthesis, purification methods, instrumentation usage, and data analysis. In addition, a Kugelrohr was acquired and was utilized extensively. It made purification safer and more manageable.