57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND1051458-ND10: New Insights on High Performance Anodes for Lithium-Ion Batteries
Reza Shahbazian Yassar, PhD, Michigan Technological University
The current challenge in the development of high power and high capacity Li-ion batteries is directly related to the material chemistry used in anodes and cathodes. A substantial progress can be made in this direction if we can replace the carbon-based anodes with higher capacity materials such as silicon. However, silicon does not remain passive when Li-ions enter its crystal structure and makes multiple alloying phases with lithium. The major challenge comes from the formation of these new Li-Si alloys because they can induce volume expansions more than 200-300%, and ultimately mechanical fracture during charging-discharging cycles. A better understanding on the mechanisms of the phase transformations, stress-strain development, and chemical gradient development during Li-ion insertion/deinsertion is of prime interests for the battery research community.
In Year 1, a post-doctoral researcher was hired to fully focus on this research topic. The post-doctoral researcher was recruited from China and joined the laboratory in July 5th of 2012. This research scholar has several years of research experience working with in-situ STM-TEM setup during his PhD and therefore should be able to follow up the in-situ battery tests that we conducted during the past year with the help of 2 PhD students in my group. These PhD students studied Si nanostructures as anode for Li-ion battery and were successful in publishing two journal articles. One of the journal articles is published in ACS Nano, which has the impact factor > 9. The second article is published in Applied Physics Letter with impact factor > 3.
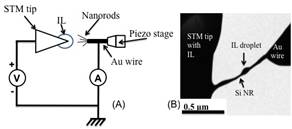
In Year 1 of our research project, the Li insertion/extraction studies of Si nanostructures were conducted using an in-situ electrochemical setup that operated inside a chamber of a transmission electron microscope (TEM). Ionic liquids was used as Li-enriched media and Si nanostructures were subjected to electrical potential using a scanning tunneling microscopy (STM) to simultaneously image the atomic structure of Si matrix during electrochemical reactions. Particular attention was given to monitor LixSi phase transformation and fracture of Si nanostructures to determine a size scale regime that electrochemical-induced fracture does not happen.
In our journal paper published in ACS Nano, we discussed in situ electrochemical lithiation and delithiation processes inside a nanobattery consisting of an individual amorphous Si nanorod and ionic liquid. Direct formation of the crystalline Li22Si5 phase due to the intercalation of Li ions was observed. In addition, the role of the electrolyte-nanorod interface was examined. It was observed that the lithiation of Si nanorods is dominated by surface diffusion. Upon the delithiation process, partial decomposition of Li22Si5 particles is observed which can explain the irreversible capacity loss that is generally seen in Si anodes. This study shows that the radial straining due to lithiation does not cause cracking in nanorods as small in diameter as 26 nm, whereas cracks were observed during the lithiation of 55 nm Si nanorods.
Figure 1 (a) Schematic of STM holder experimental setup. (b) Shows the low-magnification image during the lithiation experiment.
In our second journal article published in Applied Physics Letters, we studied the formation of lithium dendrite/fibers during charging-discharging cycles to address the safety issue of nanobatteries. Our results show that during the lithiation process, the lithium ions nucleate at the interface of anode and electrolyte and then grow into fibers. These fibers grew parallel to the direction of the applied electric field. Such observations can assist the nanoscale design of better electrodes and electrolyte materials needed for safe and high power Li-ion batteries.
Figure 2 (a) Black arrows indicate an individual NR surrounded by IL. (b) Arrows indicate the formation of Li islands on the NR. (c) Represents the growth of Li fibers. (d) The formation of kinks and growth of Li fibers are marked by black arrows.
In addition to these publications, we have presented our Li-ion battery research funded by ACS PRF in several international conferences including the annual meeting of The Minerals, Metals, and Materials Society (TMS) in March 2012, Materials Research Society (MRS) spring and fall meetings in April 2012 and Dec 2011, Challenges in Alternative & Renewable Energy (MCARE) Conference in Feb 2012, and The 36th International Conference & Exposition on Advanced Ceramics & Composites (ICACC) in Jan 2012.
The future studies will focus on better understanding the nature of chemical gradients and phase transformation mechanisms during multiple charging and discharging cycles. We also like to understand the effect of crystallographic orientation on the electrochemical-induced phase transformations. We have made agreement with the University of Illinois at Chicago to use their newly installed aberration-corrected scanning transmission electron microscopy (STEM) and the electron energy loss spectroscopy (EELS) capabilities. With these new capabilities, we should be able to detect various atomic-scale mechanisms that are triggered by Lithium during entering in silicon crystal structure.