57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR349334-UR3: Photocatalytic Dechlorination of Chloroalkanes in Hydrocarbon Mixtures
Patrick E. Hoggard, Santa Clara University
The goal of this PRF-funded research is to develop catalytic methods to remove chloroalkanes impurities from petroleum by photochemical degradation. To be practical, the catalyst must act heterogeneously. In our studies thus far we have used cyclohexane as a stand-in for petroleum and chloroform as the chloroalkane. We have followed the photodegradation process through the accumulation of decomposition products during irradiation.
The proposed work was based on two hypotheses. One was that metal complexes irradiated by visible or near UV light would be used to create radicals in solution, by any of several mechanisms. For example, using a chlorometallate complex (abbreviated MCln+), the following sequence can take place:
The trichloromethylperoxy radicals go on to produce phosgene, which can be converted to CO2 and HCl by exposure to water.
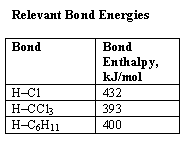
The second hypothesis was that the photodegradation would occur even at very low chloroform concentrations, because the decomposition would still take place even if hydrogen were abstracted from the hydrocarbon rather than chloroform itself. This is because the resulting alkyl (or aryl) radical would to abstract hydrogen from CHCl3, an exothermic process (see Table)
It is clear that in order for the degradation to proceed as in Eq. (3),
O2 will have to be carefully controlled. If the O2 concentration is too high, the reaction with cyclohexyl radicals may compete with the desired hydrogen abstraction, Eq. (5);
If the O2 concentration is too low, the formation of CCl3OO, as in Eq. (3), will be too slow compared to chain termination steps, particularly Eqs. (7) and (8).
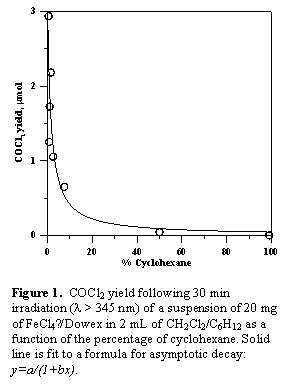
We have found several heterogeneous catalysts that promote the photodecomposition of chloroform: (a) CuCl42- supported on a Dowex anion exchange resin, (b) FeCl3 supported on silica gel, (c) FeCl4 supported on Dowex, and (d) unmodified mesomorphous silica, MCM-41. We have not yet tested the tetrachlorocuprate with cyclohexane. MCM-41 loses its catalytic activity upon addition of even small amounts of cyclohexane. FeCl3/silica also loses activity rapidly upon addition of cyclohexane, though not as rapidly as MCM-41. Photocatalysis by FeCl4 on Dowex slows down upon the addition of cyclohexane, but not nearly as drastically as with the other two catalysts. The decline in yield with the percentage of cyclohexane is shown in Figure 1, which shows an experiment with dichloromethane rather than chloroform (we have not yet done the chloroform experiment).
While this appears to be anything but promising, it is actually considerably better than the other catalysts, showing some photodecomposition of dichloromethane even in a 50-50 mixture with cyclohexane. Considering that the effect of O2 on this system has not yet been studied, there is a good chance that yields can be significantly improved.
We also examined one homogeneous catalyst, the hexachlororuthenate(IV) ion, which quite effectively catalyzed the photodecomposition of chloroform, but even a small amount (0.5%) of cyclohexane completely shut the reaction down.
The catalyst in our current arsenal that has not been tested at all, CuCl42- on Dowex, causes photodecomposition at approximately 50 times the rate achieved by the other heterogeneous catalysts. Thus, even if presented with a profile similar to that in Figure 1, we may find this to be a usable system in cyclohexane containing a small amount of chloroform.