57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI1051019-DNI10: Development of Novel Facilitated Transport Membranes Consisting of Structure-Tunable Molecular Cages for Efficient Olefin/Paraffin Separation
Wei Zhang, PhD, University of Colorado (Boulder)
Introduction:
Recently discrete organic polyhedrons emerged as promising candidates for low density organic porous materials for gas storage and/or separation applications. These covalent organic polyhedrons (COPs) possess internal cavities, so-called intrinsic porosity, as well as extrinsic porosity created by packing of these molecules in the solid state. Our group has been interested in exploring the possibility of applying low-density COP compounds and their cross-linked framework materials for gas separation, especially for separation of CO2/N2. With the advent of dynamic covalent chemistry, chemists have been able to gain facile access to a wide variety of molecular cages. Compared to supramolecular assemblies based on metal-ligand coordination or hydrogen bonding, chemically and thermally robust covalent organic polyhedrons (COPs) have been less explored, and there still exist significant challenges to synthesize COPs of precise dimensions and shape with surface functionalities, which is of great importance to tune the bulk material properties. We have synthesized a series of COP molecules through dynamic imine metathesis and explored the design principles of building blocks for the successful syntheses of COPs. We also studied their gas adsorption properties.
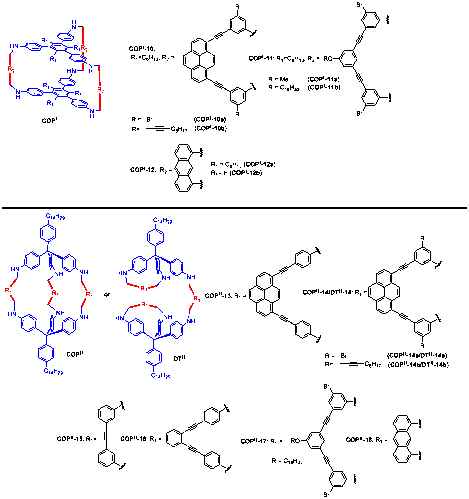
Exploring the design principles for shape-persistent COPs: a series of dialdehyde compounds (1-7) were synthesized and reacted with the complementary triamines (8-9, either planar or pyramidal with a 109.5o vertex) in a 3:2 ratio to explore the structural requirements on the building blocks for the successful construction of shape-persistent, covalent organic polyhedrons (COPs). Structural variations in the building blocks included the distance and angle between the two reactive sites (aldehyde or amine functional groups), and the absence/presence of solubilizing chains. The results are summarized in Table 1. In all the attempted syntheses of COPs, the imine condensation/metathesis was conducted at room temperature (rt) under acid catalysis. The concentrations of the triamine and dialdehyde building blocks are 3.0 and 4.5 mM, respectively. We found the concentration is important: high concentration leads to fast initial condensation to form insoluble intermediate oligomeric species; low concentration leads to very sluggish reaction. Upon formation of the imine-linked COPs, diisobutylaluminum hydride (DIBAL-H) was added as an efficient reducing agent to reduce the imine bonds to the amines at various temperatures. As shown in table 1, we were able to isolate pure trigonal prisms (COPI-10a, COPI-10b, COPI-11a, COPI-11b, COPI-12a, COPI-12b) and elongated molecular triangular bipyramids (COPII-13, COPII- 15, COPII-16, COPII-17, and COPII-18) in various yield (11 % → 94 %).
Chart 1. Triamine and dialdehyde building blocks for COP synthesis.
Figure 1. COP/DT structures observed in this study.
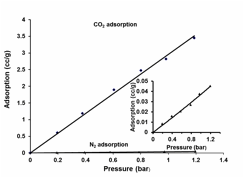
Gas adsorption study: Under the STP (1 bar, 20 oC) condition, trigonal prismatic cages COPI-16b, COPI-17a and COPI-17b showed a high selectivity of up to 138/1 (CO2/N2), thus showing great potential in carbon capture applications. The gas adsorption behavior of the elongated triangular bipyramidal cages (COPII) containing the new pyramidal triamine building blocks (9) was investigated by using cage COPII-15 as a representative example. We measured the gas adsorption isotherms of amorphous desolvated sample of COPII-15 using the custom-built instrument for low-adsorption-capacity materials. The gas adsorption isotherms (Figure 2) show
Table 1. Syntheses of COPs via imine condensation/metathesis followed by reduction.
entry | triamine | dialdehyde | solvent | COP product | Yielda |
1 | 8b
| 1
| TCB | ND | NA |
2 | 8b
| 2a
| TCB | COPI-10a
| 23 |
3 | 8b
| 2b
| CHCl3 | COPI-10b
| 83 |
4 | 8b
| 3
| CHCl3 | ND | NA |
5 | 8b
| 4
| CHCl3 | ND | NA |
6 | 8b
| 5
| CHCl3 | ND | NA |
7 8 | 8b 8b
| 6a 6b
| CHCl3 CHCl3 | COPI-11a COPI-11b
| 21 46 |
9 | 8b
| 7
| CHCl3 | COPI-12a
| 74 |
10 | 8a
| 7
| CHCl3 | COPI-12b
| 75 |
11 | 9
| 1
| TCB | COPII-13
| 78 |
12 | 9
| 2a
| TCB | COPII-14a/ DTII-14ab | trace |
13 | 9
| 2b
| CHCl3 | COPII-14b/ DTII-14bb | trace |
14 | 9
| 3
| CHCl3 | COPII-15
| 31 |
15 | 9
| 4
| CHCl3 | COPII-16
| 94 |
16 | 9
| 5
| CHCl3 | ND | NA |
17 | 9
| 6a
| CHCl3 | COPII-17
| 11 |
18 | 9
| 7
| CHCl3 | COPII-18
| 69 |
ND = Not detected, NA = Not applicable, a Isolated yield, b Species with molecular weight corresponding to COPII/DT was observed on the MALDI mass spectra.
Figure 2. CO2- and N2-adsorption isotherms at 20 oC for COPII-15. Inset: N2-adsorption.
a comparable adsorption capacity and CO2/N2 selectivity to COPI derivatives, with an adsorption capacity of 2.96 cc/g for CO2 and 0.037 cc/g for N2, and an ideal adsorption selectivity of 80/1 under the STP condition, thus showing great potential, together with other recently developed COPs, for carbon capture applications.
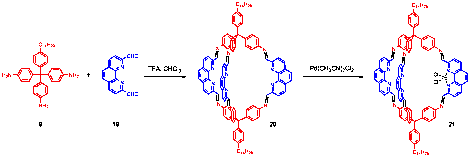
Synthesis of phenanthroline-based COP: Given the knowledge learned from the above systematic study, we successfully synthesized a phenanthroline-based shape-persistent COP (20) from triamine 9 and dialdehyde 19 (Figure 3). Cage 20 exhibits a CO2 adsorption capacity of 14.5 cc/g, which is almost 5 times higher than that of COPII-15. However, the selectivity in CO2/N2 adsorption dropped to 16.5/1. Currently we are preparing metallated COPs 21 by reacting 20 with PdCl2 or AgOTf salt. Eventually we will integrate the metal-coordinated COPs (21) into membranes to achieve highly energy-efficient light hydrocarbon separation.
Figure 3. Synthesis of phenanthroline-based, metal-coordinated COP 21.
Summary. The experimental results showed that both the angle and the directionality (e.g., two aldehyde groups locked in the same direction) of the functional groups as well as their solubility in the reaction medium are very important to high-yielding molecular cage synthesis. The alignment of aldehyde and amino groups in the precursors with an appropriate angle is highly desired. The presence of certain rotational freedom in the building blocks can decrease the angle strain built along the cage formation, thus facilitating the cage synthesis.