AmericanChemicalSociety.com
Reports: UR4 49182-UR4: New Directions in Understanding Conformational Preferences of 1,2-Disubstituted Ethanes
John D. Roberts, California Institute of Technology
What was accomplished in 2010 and plans for 2011 on Conformational Analysis?
First, a primer of factors important in conformational analysis, which includes steric, electrical, solvation with protic solvents, such as water, with hydrogen-donating groups, generally disfavoring formation of intramolecular hydrogen bonds of monoanions of dicarboxylic acids. Aprotic solvents, like DMSO or THF, do not possess hydrogen-donating groups. However, aprotics can provide help in forming intramolecular hydrogen bonds with monoanions of dicarboxylic acids. How? An anion dissolved in protics or aprotics, requires stabilization of its anionic charge by solvation, which in protics involves forming hydrogen bonds to anions, particularly with water. But, while aprotics like DMSO can often stabilize positive ions, they are very poor for anions. But stabilization of monoanions, is possible by anionic carboxylate binding to unionized carboxyl in the same molecule. Structural limitations for the formation of such intramolecular hydrogen bonds include steric hindrance, ring size, stereochemistry and the acid strengths.
1. Studied conformational preferences and hydrogen bonding of 3-guanidinopropanoic acid (GPA).
With dibasic acids of type, X-CH2-CH2-Y, with X and Y as acidic groups, the X and Y acid constants, K1 and K2, are of major interest to changes in conformational preferences with pH. In titrations of a diacid, X-CH2-CH2-Y, where X and Y are acidic groups, the K1/K2 ratio is touted as a positive indicator of intramolecular hydrogen bonding in water, if K1/K2 > = 104. Why? Because the strength of a hydrogen bond will be greater, the more powerful the proton-donating power of the acid, as measured by K1. Then, a strong hydrogen bond decreases K2 making it harder to remove the hydrogen bond proton by losing its stabilization in the second ionization.
The range of observed K1/K2 values in water is substantial. Succinic acid, small, 24; fumaric acid, 100, with neither monoanion showing evidence for intramolecular hydrogen bonding. However, K1/K2 for maleic acid = 7 x 104 and for 2,3-5,6-didehydrobicyclo[2.2.1]octane-2,3-dicarboxylic acid = 2.8 x 106. Both monoanions form strong intramolecular hydrogen bonds.
GPA has K1/K2 = 1010, a large value in water, seemingly ripe for hydrogen bond formation. Nonetheless, the -CH2-CH2- proton coupling constants from pH 1 to 13 are essentially constant and hardly indicative of hydrogen bonding.
To enhance conformational preferences, one chooses a solvent of greater capability for hydrogen bond formation than water. Here, DMSO is excellent. Why? Best is to have a relatively large K1 and here DMSO is fine, because acid ionization is facilitated by transferring a proton in the first ionization step to the negative oxygen of the DMSO's S+-O- dipolar bond. However, addition of a proton to the DMS+-O- forms the acid's monoanion. But, in the second ionization, DMSO, like other aprotics, is a poor solvator of anions, so K2 is reduced by DMSO not stabilizing anions by solvation, as possible in water by hydrogen bonding to water protons. The smaller K2 is, he larger K1/K2 becomes. Guanidino groups are exceptionally strong organic bases, and their protonated forms are very weak acids, which accounts for K1/K2 = 1010 for GPA for water solution and may make hydrogen bonding unfavorable.
With GPS in DMSO, K1/K2 is larger, 1013. The corresponding conformational preference changes with pH show only a 2-4 %shift toward minor hydrogen bonding.
Next summer, general limits to intramolecular hydrogen bonding will be investigated for large K1/K2, with different structures and solvents.
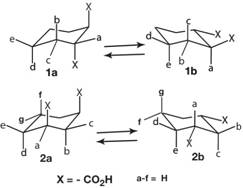
2. Do cyclohexanedicarboxylic acids show similar conformational behavior as for succinic acid and related acids? Thus, monoglutarate lacks evidence in solvents studied for intramolecular hydrogen bonding between its carboxyl groups with a -CH2-CH2-CH2- connector, but this seems a possibility for a 1,3-cyclohexanedicarboxylic 2a monoanion to form such a hydrogen bond. Also does 1,2-cyclohexanedicarboxylic acid behave like succinic acid.
However, carboxylic acid groups on cyclohexane rings are believed more stable (~2 kcal/mol) equatorial than axial. Consequently, determination of populations of diequatorial (ee) 1a and 2b and diaxial (aa) 1a and 2a conformers present in trans-1,2- and cis-l,3-cyclohexanedicarboxylic acids and their salts in water and DMSO, was needed to test the notion that diaxial conformations, (normally assumed to be quite
insignificant) might be favored depending on solvent properties and ionization state.
The conformations in play are 1 being trans-1,2- and 2 being cis-1,3-cyclohexanedicarboxylic acids.
Looking at 2a, the potential to form an intramolecular hydrogen bond can be seen from the drawing where a intramolecular hydrogen bond from monoanion of 2a might be formed as below if the carboxyls face one another so that the carboxylate group on the right can hydrogen bond to the carboxyl group on the left.
Of course, a more or less reasonable looking model does not necessarily correspond to experimental reality, particularly when depicting a hydrogen bond, has both carboxyl- and carboxylate-to-ring carbon bonds far from pure axial. Obviously, experimental evidence is needed.
Vicinal proton-proton NMR coupling constants showed strong preference for
diequatorial conformers (>90% eel) in water and DMSO for both diacids and their
salts, except for trans-1,2-dianion in DMSO, which was surprisingly found to be
substantially diaxial (55% aa). K1/K2 was <104 for both diacids in water, which militates against intramolecular hydrogen bonding. The same for the cis-1,3-diacid in DMSO.
However, in DMSO, the trans-l,2-diacid had K1/K2 = 2 x 106, so its monoanion should form a strong intramolecular hydrogen bonding and this conjecture is supported by H-H coupling constants.
In summary, the trans-1,2-diacid mimics succinic acid in both water and DMSO, so at the water K1, K2 equivalence point, neither monoanion is hydrogen bonded. However, in DMSO, the trans-1,2-diacid and succinate monoanion are both hydrogen bonded.
Unexpected was the dianion being ~ 55% diaxial, despite the poorness of DMSO to stabilize anions. A similar preference is found for succinate dianion, but not in water or simple alcohols, where trans is favored. Presumably, the negative charges interact with each other to generate sufficient stabilization energy to overcome the expected preference for the diequatorial 1b.
Copyright © American Chemical Society