AmericanChemicalSociety.com
Reports: DNI10 49976-DNI10: 3-D Quantification of Catalysts in a Reducing Environment
Ilke Arslan, PhD, University of California (Davis)
The first year of my ACS PRF DNI grant has been extremely fruitful on many levels. As the first grant of my career as a Professor, it enabled me to take on a student to perform the catalysis research I am passionate about. For the first school year, my student, Shaun Orr, received a fellowship from the department for his stipend and school fees. However, due to the nature of the research in this proposal, user fees are required for electron microscope time. This grant allowed Shaun time on the microscope to be trained and to start his experiments, and paid his salary over the summer. Further, our department has a one year teaching assistant (TA) requirement. Therefore, this grant alone has made it possible for me to mentor a student throughout his whole Ph.D. This has had a tremendously positive impact on my early career.
Furthermore, having this funding in my first year at UC Davis has helped me to establish a research group that performs detailed experimental studies. As discussed more in the next paragraph, learning electron microscopy is not easy and takes the whole first year of graduate students' careers. This grant allowed me to establish a group structure for mentoring my students. Also, it provided me with the means to continue my outreach activities, the most recent of which is to participate in the NSF-funded Portal to the Public program by giving demonstrations about my research and nanoscience to kids and the public at the Explorit Science Museum here in Davis, CA. All of these important opportunities afforded by this DNI grant assisted in my selection this year to receive a Presidential Early Career Award for Scientists and Engineers (PECASE). (It should be noted that the PECASE is for a completely different project: for the advanced characterization of energy and hydrogen storage nanomaterials used in technologies critical to National defense and homeland security).
This proposal aims to provide some of the first insights into the fundamental nanoscale mechanisms controlling the Fischer-Tropsch (FT) process by using a combination of 3-D imaging and in-situ/ex-situ gas experiments in the scanning transmission electron microscope (STEM). Fischer-Tropsch (FT) catalysts are being researched as one aspect of the solution to sustainable renewable resources because they are used to create a petroleum substitute from carbon monoxide and hydrogen, also known as synthesis gas, which can then be used to create a synthetic fuel. For the first year of the project, Shaun has spent the majority of the time getting trained on the advanced and complex electron microscopy techniques. He has had to take three quarters (one year) of classes on the theory and practice of electron microscopy, trained on the basics of how to use an electron microscope, then spent the summer gaining experience on how to perform the advanced techniques of STEM tomography, reconstructions and visualization in 3-D, and ex-situ gas experiments. Throughout the year, he spent time learning about FT catalysts and familiarizing himself with the current literature.
Using STEM tomography, the 3-D morphology of FT catalysts can be examined with a resolution of ~1 nm in all three spatial dimensions. A series of images are taken, typically in one degree increments, over a tilt range of usually +70° to -70°, although the higher the tilt range and the larger the number of images, the better the 3-D resolution. These images are then aligned and reconstructed using commercially available reconstruction algorithms (such as Inspect 3D), and displayed using visualization software (such as Avizo).
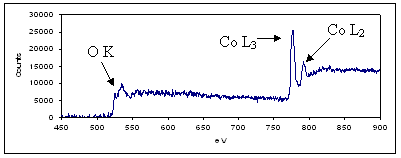
This technique will be applied here initially to two systems: an unpromoted and promoted (with Pt) Co on Al2O3 FT catalyst before and after reduction. The reduction is performed using a Fischione ex-situ vacuum transfer holder. Using this holder, the catalysts are imaged before reduction in the STEM, transferred to a reaction chamber for reduction using a mixture of gases (5% H2/95% N2) with heating provided by a CO2 laser, and transferred back to the STEM without exposure to air after reduction. Using electron energy loss spectroscopy (EELS), the oxidation state of the Co can be ascertained by examining the fine structure of the O K-edge and the peak ratios of the Co L-edges.
The preliminary results are shown in the figures below. Figure 1 shows three STEM images from the tilt series of the cobalt oxide on an alumina support at various tilt angles acquired using STEM tomography (full tilt range is -70° to +65°) for the unpromoted catalyst. It is evident that the cobalt oxide is distributed throughout the support in the form of clusters fairly uniformly. However, as the Co in the FT catalyst is not active when oxidized, STEM tomography needs to be performed on the reduced FT catalyst. To ensure that the Co is reduced, EELS spectra of the catalyst after being reduced in the reaction chamber will be compared to the spectrum of the oxidized catalyst (Figure 2). Although complex, this first data set shows the feasibility of the experiments, and shows that factors such as beam damage or contamination in the microscope are not present and will not affect the results.
With the “control” data acquired both morphologically and spectroscopically, the next step is to repeat these measurements after reduction. A further step after this 3-D ex-situ reduced analysis is to do a reduction in-situ and watch the catalyst in real time as it is reduced. This will be performed in year 2 of the project as the new in-situ holder is due to arrive in January 2011. The structure-property relationships of the active catalyst can then be illuminated fully.
Figure 1. STEM images of FT catalyst at various tilts: (a) -70°, (b) -30°, and (c) +10°
Figure 2. EELS spectrum of the FT catalyst before reduction showing the O K-edge and Co L3,2-edges.
Copyright © American Chemical Society