AmericanChemicalSociety.com
Reports: ND1 48955-ND1: Design and Synthesis of Novel Chiral Clefts and Helical Structures
Jeffrey Winkler, PhD, University of Pennsylvania
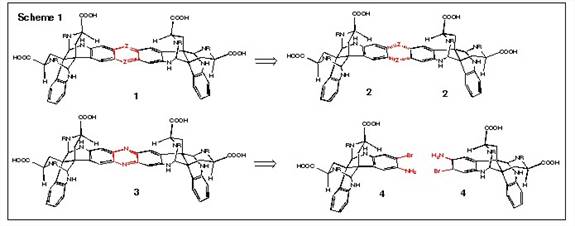
This project is directed toward the design and synthesis of chiral clefts and helical structures. We envisioned that a dimeric calycanthine 1 could be formed from two monomeric units of calycanthine 2 via an aryl ligation process that is indicated by the two “Z” groups in 1 and 2. We report herein our progress in achieving this goal. We have focused our efforts on the use of nitrogen as the “Z” group, as shown in 3, so that the ligation reaction involves the formation of a phenazine. We reasoned that the most efficient process for phenazine formation would involve a double Buchwald-Hart-wig coupling reaction from two molecules of 4 (Scheme 1).
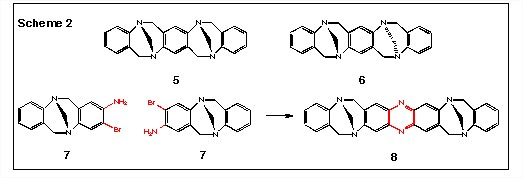
This reaction could also be applied to an elegant solution to the problem of ligating monomers of Troger's base, i.e., 7, as shown in Scheme 2. The synthesis of 5 involves the reaction of one equivalent of 1,4-diaminobenzene with two equivalents of aniline and six equivalents of formaldehyde. However, under these conditions, both the syn product 5 and the anti product 6 are formed. In an effort to effect the selective formation of 5, we wanted to ligate two equivalents of homochiral 7 to give 8, the phenazine analog of 5.
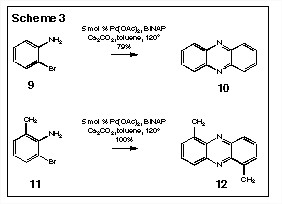
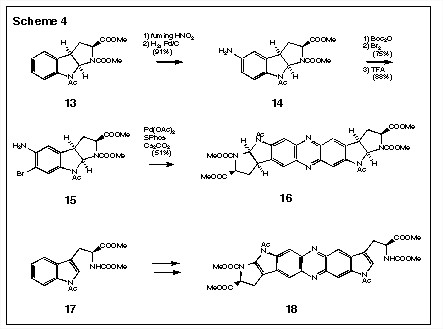
We have recently completed a model study for this reaction and we indeed find that the simple reaction, i.e., of 9Ý 10 and 11Ý 12, proceeds efficiently to give the oxidized phenazine product directly (Scheme 3). The application of this approach to the ligation of tryptophan monomers leads to the formation of compounds like 16 and 18 (Scheme 4).
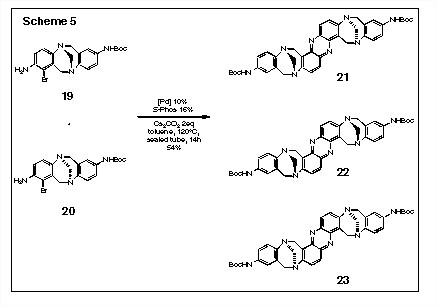
The application of this methodology to the ligation of Troger's base has recently been achieved in our laboratory. We have demonstrated that the double Buchwald-Hartwig reaction of the racemic mixture of 19 and 20 leads to a statistical mixture (ca. 2: 1: 1) of 21, 22, and 23. However, reaction of homochiral 19 under the same reaction conditions yields only one antipode of the phenazine product, i.e., 22 and analogous reaction of 20 generates only 23. The application of this methodology to more complex systems and to the generation of helical structures is currently underway in our laboratory.
The financial support of the PRF for this program has allowed us to accumulate this body of preliminary data and grant applications to both DOE and the NSF are being prepared. The PRF funds have supported a very talented high school student, Eli Bogom-Shanon, who is currently a freshman studying chemistry at Columbia, and two postdoctorals, Barry Twenter and Thomas Gendrineau, both of whom will soon move to careers in chemical industry.
Copyright © American Chemical Society