AmericanChemicalSociety.com
Reports: DNI7 49249-DNI7: Benzobisazoles as Modular Building Blocks for Novel Organic Electronic Materials
Malika Jeffries-EL, Ph.D, Iowa State University
As a result of their semiconductor like optical and electronic properties pi-conjugated organic materials are sought after to replace inorganic materials in photovoltaic cells (PVC)s. These devices can benefit from the many attractive features of organic materials such as ease of processing and the ability to tune their electron properties for specific applications through chemical synthesis. However, the low power conversion efficiencies of polymer photovoltaics limit their utility. The objective of this PRF-funded research project is to develop new electron accepting conjugated polymers for use in all polymer photovoltaic cells. To accomplish this task, we have combined experimental and theoretical methods to evaluate the impact of substitution on the central benzene ring (positions 4 and 8) of benzo[1,2-d;4,5-d'] bisoxazole. Using this approach we seek to innovate layered polymer photovoltaic cells by creation new materials with tunable polymer HOMO and LUMO levels. The final goal is to develop materials with LUMO levels that are suitable for use as electron acceptors in organic photovoltaic cells.
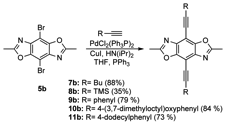
In the first year of this project, we set out to predict which types of substituents would be beneficial and to develop efficient pathways for their synthesis. To date, we have performed theoretical and synthetic investigations on nine different model compounds based on 2,6-dialkyl substituted benzobisoxazoles. Our results demonstrate that electron-deficient substituents such as halogens do not impact the electronic properties of the benzobisoxazoles. However they do serve as excellent reactive groups for coupling other substituents onto the ring. Using either Suzuki or Sonogashira coupling reactions we were able to extend the conjugated across position 4- and 8- by attaching substituted alkynes or benzene rings, Figure 1. As a result of these substitutions we see a reduction in the band gap of the resulting compounds as a result of a decrease in both the LUMO and HOMO. The extent of these changes varies depending on the types of groups attached. Using this approach we are able to tune the LUMO and HOMO over a 1 eV. Furthermore, the experimental results show excellent correlation to the theoretical values demonstrating that we can use theory to predict and optimize the energy levels of these materials.
Figure 1.
Synthesis of 4,8- substituted benzobisoxazoles
HOMO (eV) LUMO (eV) Egopt (eV) Experiment Theory Percent Error Experiment Theory Percent Error Experimentb Theory Percent Error 4b -5.90c -6.04 2.4 -1.40 -1.01 28.1 4.5 (275) 4.70 4.4 5b -6.50c -6.20 4.6 -1.30 -1.45 11.7 5.2 (237) 4.42 15.0 6b -6.50c -6.30 3.1 -1.20 -1.47 22.8 5.3 (234) 4.52 14.8 7b -5.93d -5.40 8.9 -1.23 -1.43 16.1 4.7 (263) 3.84 18.2 8b -6.09d -5.70 6.5 -1.49 -1.81 17.9 4.6 (269) 3.74 18.7 9b -5.92d -5.30 10.5 -1.42 -1.97 32.9 4.5 (275) 3.16 29.8 9b* -5.67 4.3 -1.67 17.3 3.76 16.5 10b -5.70d -4.97 12.8 -1.10 -1.74 58.2 4.6 (270) 3.03 34.1 10b* -5.54 2.9 -1.54 40.4 3.41 25.9 11b -5.78d n/a n/a -1.45 n/a n/a 4.3 (290) n/a n/a Table 1. An experimental and theoretical comparison of the electronic
properties of BBO model compounds We have also synthesized
polymers by copolymerizing monomers derived from 1a, with alkyl
thiophenes. The resulting polymers have poor solubility as a result of the
halogens that have been appended onto the middle ring. However, the predicted
HOMO and LUMO levels for these polymers show excellent correlation with the
results obtained from cyclic voltammetry, with a margin of error of around 6%.
Further supporting the validity of our methods. The data for this polymer is
shown below. We are currently synthesizing new polymers based on the building
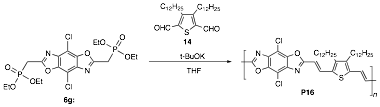
blocks with extended pi-conjugation. Figure 2. Synthesis of
poly(thiophenevinylene) benzobisazoles, and
related data
HOMO (eV) LUMO (eV) Egopt (eV) Experiment Theory Percent Error Experiment Theory Percent Error Experimentb Theory Percent Error P16 -5.38 -5.43 0.93 -3.30 -3.32 0.61 2.05 2.25 5.0 The additional positive outcomes of the
research are the impact it has had on this young research group and its
personnel. Thus far 2 graduate students and 1 undergraduate student were
supported by this grant. One of the students involved in this project presented
his research at the International Symposium on pi-Functional Materials (FPi-9).
We also collaborated with Dr. Aimee Tomlinson of North Georgia College and
State University on the theory portion of the project, further increasing
undergraduate participation. Additionally, the PI has given several invited
talks at universities and 1 invited talk at the American Chemical Society
National Meeting (Washington, DC). We are also currently preparing a manuscript
on this research.
Copyright © American Chemical Society