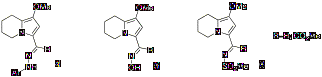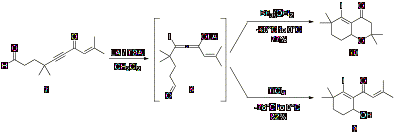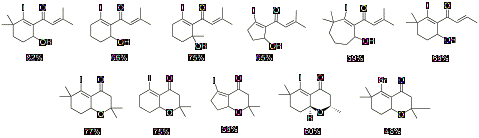AmericanChemicalSociety.com
Reports: AC1 47919-AC1: Development of Catalytic Asymmetric Methods for the Synthesis of Pyrrolidine Derivatives via Hydrogenation of Substituted Pyrroles
Alison Frontier, University of Rochester
Part 1: Stereoselective synthesis of substituted pyrrolidines
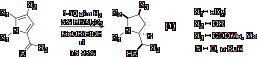
Part of the funding provided by this grant focused on the heterogeneous catalytic hydrogenation of highly substituted pyrrole systems. These aromatic systems can be fully reduced with excellent diastereoselectivity to afford functionalized pyrrolidines with up to four new stereocenters (equation 1). It is likely that the reaction is a two-step hydrogenation sequence, and that initial reduction of the C=X bond provides a stereocenter that directs the subsequent reduction of the pyrrole.
Studies focused
on expanding the scope of the reaction to increase its synthetic value: in
order to develop it into 1) a method with broad substrate scope and 2) a
practical chemical process. With respect to the first goal, a study focused on
expanding the scope of the reaction to nitrogen-containing compounds, which
represent an important class of biologically relevant targets. Substrates of types 4, 5
and 6 were chosen for synthesis
and hydrogenation studies.
Although these studies are not yet complete, we are beginning the
process of understanding the relationship between structure and hydrogenation
efficiency. For example, with
substrate 4 (R=H), we found that
hydrogenation was smooth and that the N-N bond was not cleaved under the
reaction conditions.
In
pursuance of the second goal, we are preparing an Organic Synthesis article describing the method for use in practical
contexts. The chemistry is being
tested on large scale and documented carefully for this article, so that others
may apply the hydrogenation with confidence for their own purposes. Table 1 describes the current scope of
the process, and summarizes typical reaction conditions for its practical use
in synthesis of racemic pyrrolidine compounds. Using this heterogeneous hydrogenation method, highly substituted
pyrroles can be fully reduced with excellent diastereoselectivity to afford functionalized
pyrrolidines with up to four new stereocenters. The method is effective for a series of pyrrole a-ketoesters, as shown in Table 1. Typically,
the hydrogenation requires 10 atm of H2, 5% (w/w with substrate)
rhodium and 12-24 hours of reaction time to ensure complete conversion to the
pyrrolidine products 3. Furthermore, it
was found that when the hydrogenation in entry 1 was conducted using 1% (w/w
with substrate) of Rh/Al2O3 on 0.8 mmol scale, reaction
time, selectivity and yield were comparable. If this finding is general, which will be investigated in
the next stages of the research, it could represent a significant improvement
in cost-effectiveness for the process. Table 1. Stereoselective
Reduction of Bicyclic Pyrrolesa
entry pyrrole ratio % yield productc 1b >20:1 95 2 >20:1 90 3 >20:1 75 4 >20:1 91 5 9:1 91 6 >20:1 91 7 >20:1 90 (a) Reaction conditions: 0.1 mmol substrate, 10 atm H2,
5% Rh (w/w with substrate), room temperature, MeOH or EtOH, 12-24 h; (b)
Reaction conditions:
0.8 mmol substrate, 1 atm H2, 1% Rh (w/w with substrate), room
temperature, EtOH, 6h; (c) Stereochemical assignments are proposed based on the
structure of 3a. In summary, the scope and limitations of the reactions
with respect to practical applications were better defined over the course of
this grant: optimal hydrogenation conditions were identified and expansion of
substrate scope was studied. The
testing of asymmetric hydrogenation catalysts has also been initiated, although
success in this area has been elusive in the short time spent exploring it.
Part 2: The Development of
Tandem Annulation Processes via Allenolate Derivatives Funds
during this granting period have also been directed toward another project in
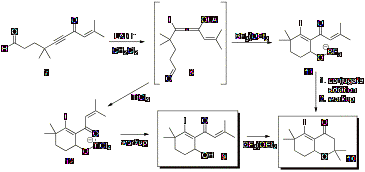
the laboratory, focused on a new annulation method. As shown in Scheme 1, β-iodoallenolate intermediates,
such as intermediate 8, can be used in
annulation reactions to afford products such as cyclohexenyl alcohol 9 and oxadecalin 10.
Scheme
1: β-Iodoallenolate Cyclization Optimal mono and
bicyclization conditions for alkynone 7
were determined by varying the iodide source and the Lewis acid. The results
from these reactions indicated the optimal Lewis acid and iodide source for
obtaining cyclohexenyl alcohol 9
were TiCl4 and n-Bu4NI (TBAI), and the
optimal Lewis acid and iodide source for obtaining oxadecalin 10 were BF3¥OEt2 and TBAI,
(Scheme 1). The optimal temperature for mono and bicyclization was -78°C to 0°C
and -40°C to 0°C, respectively. Furthermore, a stoichiometric amount of BF3¥OEt2
was required for the complete conversion of alkynone 7 to oxadecalin 10.
Next,
we explored the scope and limitations of both mono and bicyclization. The
products obtained from both mono and bicyclization are shown in Chart 2.
Chart
2: Mono and Bicyclization Products A variety of
cyclohexenyl alcohols and oxadecalins were synthesized in moderate to good
yields.
The
proposed pathway for the conversion of alkynone 7 to oxadecalin 10 is
illustrated in Scheme 2. Conjugate addition of the iodide to Lewis acid
activated alkynone 7 produces β-iodoallenolate
8. β-Iodoallenolate 8 would then undergo an aldol reaction to produce intermediate
11, which would cyclize to
oxadecalin 10 through a Lewis
acid triggered intramolecular oxy-Michael addition. Scheme
2: Proposed Mechanistic Pathway The
difference in the reactivity and selectivity that was observed between TiCl4
and BF3¥Et2O was likely due to the fact that TiCl4,
a bidentate Lewis acid, could be chelated to both oxygen atoms in
intermediate 12, which would prevent
bicyclization. However, bicyclization can occur with BF3¥Et2O,
a monodentate Lewis acid, because intermediate 11 is in the proper orientation to allow the
oxy-Michael addition and generate oxadecalin 10.
In
summary, we have demonstrated the β-iodoallenolate intermediates can be
used to produce a variety of cyclohexenyl alcohols and oxadecalins. The success
of this β-iodoallenolate cyclization has prompted us to explore its
utility in the total synthesis of two biologically active natural products,
phomactin A and phomactin D, which is currently underway. Significant progress toward these
syntheses has been achieved during the grant period, and a full account of this
work will be reported in due course.
Copyright © American Chemical Society