AmericanChemicalSociety.com
Reports: AC7 47774-AC7: Synthesis Characterization and Fundamental Studies of Novel Chiral Ionic Liquid and Their Polymers
Shahab A. Shamsi, Georgia State University
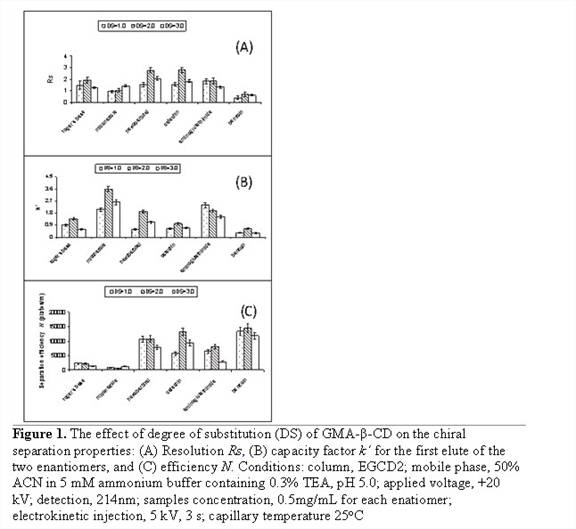
Another study was conducted to check the effect of DS on the
chiral separation property of the prepared monolithic column. Figure 1A-C shows
the separation properties It was found that GMA-b-CD-2 monolithic
column prepared with DS=2.0 provided the highest Rs and kx for most of the analytes.
For the separation efficiency N, except for miconazole, column GMA-b-CD-2
demonstrated the highest efficiency. Overall, GMA-b-CD-2 showed the best
chiral separation performance.
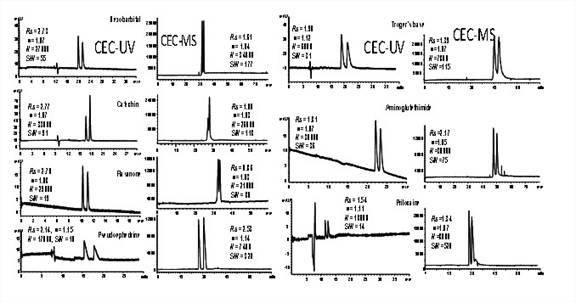
Figure 2. CEC-ESI-MS and CEC-UV separation of hexobarbital, catechin, flavanone, pseudoephedrine,
aminoglutethimide, troger's base, and prilocaine chiral compound on GMA-b-CD-2 monolithic column. Conditions: mobile phase, 50% ACN in 5
mM ammonium acetate buffer containing 0.3% TEA, pH 4.0; applied voltage, +20
kV; detection, 214nm; samples concentration, 0.5mg/mL for each racemic
compound; electrokinetic injection, 5 kV, 3 s; capillary temperature 25 oC.
Copyright © American Chemical Society