AmericanChemicalSociety.com
Reports: B7 45134-B7: Competition between Mesogens in Pyridone-Based Supramolecular Calamitic, Banana, and Discotic Liquid Crystals
Kurt N. Wiegel, University of Wisconsin (Eau Claire)
In the first year, Justin Kumpfer worked on the synthesis of pyridone-based esters. It was found that while the substitution chemistry of pyridones was difficult, the species were capable of reacting through nucleophilic acyl substitutions. Metal-based coupling reactions proved problematic, as the pyridine functionality chelated the metal from the catalyst, rendering the reaction inert. These results provided the groundwork for the rest of the project.
In the second year, David Witte synthesized a series of bis-functionalized pyridone terminated esters. Supramolecular polymers produced flexible, long-lived fibers pulled from the melt, but interestingly produced only a frustrated nematic phase observable only upon crash cooling the isotropic melt in liquid nitrogen. It is believed that the nematic phase could only be captured in this dramatic fashion because the overall structure of the assembled pyridone species would be too irregular to effectively form a mesogenic phase.
|
Figure 1: Materials for Tetra-network Study |
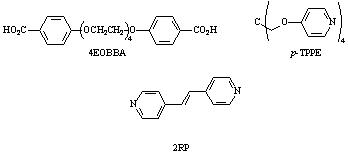 Also in the second year, a side project funded
by this grant was carried out by Jason Greuel involving the investigation of
the liquid crystalline properties of supramolecular networks. We observed the formation
of a series of mesogenic networks with increasing netpoint
inclusion. The mesogenic portions of these systems arise from a hydrogen bonded
interaction between a bisbenzoic acid
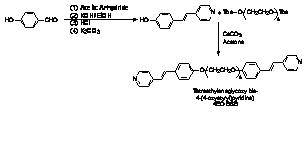
(tetraethyleneglycoxy-bis-4-benzoic acid, 4EOBBA), a rigid bispyridyl
system (1,2-bis(4-pyridyl) ethylene), and a tetrafunctionalized non-mesogenic networking agent
(tetrakis-4-pyrixyloxy methane, p-TPPE),
which is used to introduce a network and disrupt liquid crystallinity. All the
materials uses in this study are outlined in Figure 1. The networking agent was
Also in the second year, a side project funded
by this grant was carried out by Jason Greuel involving the investigation of
the liquid crystalline properties of supramolecular networks. We observed the formation
of a series of mesogenic networks with increasing netpoint
inclusion. The mesogenic portions of these systems arise from a hydrogen bonded
interaction between a bisbenzoic acid
(tetraethyleneglycoxy-bis-4-benzoic acid, 4EOBBA), a rigid bispyridyl
system (1,2-bis(4-pyridyl) ethylene), and a tetrafunctionalized non-mesogenic networking agent
(tetrakis-4-pyrixyloxy methane, p-TPPE),
which is used to introduce a network and disrupt liquid crystallinity. All the
materials uses in this study are outlined in Figure 1. The networking agent was | Figure 2: Netpoint for Tris-network Study |
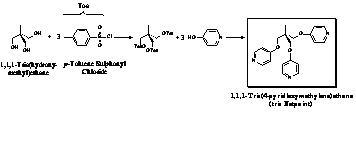 added in increasing concentrations to the system.
A smectic phase was observed in concentrations up to 8.5% of networking agent.
The nematic phase was observed until concentrations up to 22.5%, which
displayed a frustrated nematic phase, barely forming before crystallizing. At
25% and above, only melting and crystallization behavior was observed. It was
curious to note that the cut-off for any liquid crystallinity (22.5%) which correspons closely with the tetra-functionalized nature of
the crosslinking agent. This phenomenon may arise
from a statistical correlation, where at 25%, one of the benzoic acid
components would always be hydrogen bonded with one of the networking species,
inhibiting the formation of the hydrogen bond with the rigid 2RP species, and
therefore inhibiting the formation of the hydrogen bond.
added in increasing concentrations to the system.
A smectic phase was observed in concentrations up to 8.5% of networking agent.
The nematic phase was observed until concentrations up to 22.5%, which
displayed a frustrated nematic phase, barely forming before crystallizing. At
25% and above, only melting and crystallization behavior was observed. It was
curious to note that the cut-off for any liquid crystallinity (22.5%) which correspons closely with the tetra-functionalized nature of
the crosslinking agent. This phenomenon may arise
from a statistical correlation, where at 25%, one of the benzoic acid
components would always be hydrogen bonded with one of the networking species,
inhibiting the formation of the hydrogen bond with the rigid 2RP species, and
therefore inhibiting the formation of the hydrogen bond.
A further study involved annealing
these networks in the mesophase and observing a premature onset of
crystallization. This onset rose to higher and higher temperatures with
increasing netpoint inclusion. This phenomenon was
not observed in small molecule covalent or supramolecular liquid crystals. The
first study has produced a paper which will soon be submitted to Liquid
Crystals, and the second manuscript is in development. Both will have two
undergraduate authors.
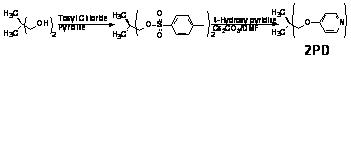
Figure 3: Netpoints for Tris- and Bis- centered Network Study Figure 4: Small Molecule Liquid Crystalline Materials Figure 5: Distonic Mesogen Forming Agents
 As the pyridone project seemed to be at an
end, producing systems barely mesogenic in nature, further investigation was
carried out on the networked systems. In order to determine if the statistical
correlation between the functionalty of the
networking agent and the disappearance of the liquid crystalline phase exists,
a tris-functionalized networking agent was
synthesized as outlined in Figure 2. The networks so created displayed smectic
phases until 15% concentration, and a complete destruction of mesogenicity (also a frustrated nematic phase) at 32%. As
a continuation of this work, a series of non-mesogenic bis pyridyl system was
synthesized- as detailed in Figure 3. This work, carried out by Jason Greuel,
Tim Andrews and Justin Wichman, involved the synthesis of the polymer-forming
agent as well as its inclusion in the macromolecular systems. It was found that
the this agent displayed liquid crystallinity when
mixed with 4EOBBA and 2RP in loadings up to 25% (10% for the presence of
smectic phases). This is markedly lower than the expected 50% based off of
previously observed statistical correlations. It is believed that the markedly
reduced melting point of the 2PD system is the principal contributor to the
retardation of the transitions. The results of this work are being compiled
into a publication which will have four undergraduate authors and will be
submitted to Liquid Crystals.
As the pyridone project seemed to be at an
end, producing systems barely mesogenic in nature, further investigation was
carried out on the networked systems. In order to determine if the statistical
correlation between the functionalty of the
networking agent and the disappearance of the liquid crystalline phase exists,
a tris-functionalized networking agent was
synthesized as outlined in Figure 2. The networks so created displayed smectic
phases until 15% concentration, and a complete destruction of mesogenicity (also a frustrated nematic phase) at 32%. As
a continuation of this work, a series of non-mesogenic bis pyridyl system was
synthesized- as detailed in Figure 3. This work, carried out by Jason Greuel,
Tim Andrews and Justin Wichman, involved the synthesis of the polymer-forming
agent as well as its inclusion in the macromolecular systems. It was found that
the this agent displayed liquid crystallinity when
mixed with 4EOBBA and 2RP in loadings up to 25% (10% for the presence of
smectic phases). This is markedly lower than the expected 50% based off of
previously observed statistical correlations. It is believed that the markedly
reduced melting point of the 2PD system is the principal contributor to the
retardation of the transitions. The results of this work are being compiled
into a publication which will have four undergraduate authors and will be
submitted to Liquid Crystals.
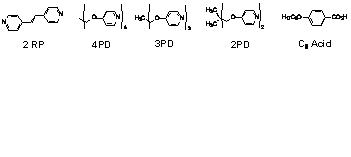 In order to determine if similar effects are
observed in small-molecule, discrete liquid crystalline systems, similar
complexes were made by Michael Zenner and Joshua Tessner
utilizing 4-octyloxybenzoic acid as the hydrogen bond donor. The materials used
in this portion of the study are outlined below in Figure 4. Complexes made
using the C8 Acid, 2RP and 4PD displayed liquid crystallinity in
loadings of 4PD up to 99% that still displayed strong liquid crystalline
characteristics. The extremely high loading of the disruptive 4PD into the
complexes that retained liquid crystallinity was very surprising- it is
surmised that the C8 acid is forming mesogenic dimers in the
presence of excess 4PD and a minimum
In order to determine if similar effects are
observed in small-molecule, discrete liquid crystalline systems, similar
complexes were made by Michael Zenner and Joshua Tessner
utilizing 4-octyloxybenzoic acid as the hydrogen bond donor. The materials used
in this portion of the study are outlined below in Figure 4. Complexes made
using the C8 Acid, 2RP and 4PD displayed liquid crystallinity in
loadings of 4PD up to 99% that still displayed strong liquid crystalline
characteristics. The extremely high loading of the disruptive 4PD into the
complexes that retained liquid crystallinity was very surprising- it is
surmised that the C8 acid is forming mesogenic dimers in the
presence of excess 4PD and a minimum
 of 2RP. 3PD and 2PD containing systems
display similar characteristics, with lower concentrations leading to the
disruption of liquid crystallinity.
of 2RP. 3PD and 2PD containing systems
display similar characteristics, with lower concentrations leading to the
disruption of liquid crystallinity.
Copyright © American Chemical Society

