AmericanChemicalSociety.com
Reports: AC3 47899-AC3: Rational Syntheses of Metallocarbohedrynes (Met-cars)
Michael C. Baird, Queen's University
Introduction
The Basic Procedures Last year's annual report
outlined our general approach to the problem, described the types of
experiments which we had carried out, and need not be repeated in detail. In
essence, we consider the C2 units to be acetylidic (C22-)
in nature and therefore the average formal oxidation state of the titanium atoms
in e.g. Ti8C12 is +1.5. Our general synthetic procedures
therefore initially involved combining sources of C22-
with titanium precursors in the +3 and +4 oxidation states in the presence of
reducing agents capable of reducing the Ti(III) and Ti(IV) precursors to the
oxidation state(s) desired.
In view of its ready availability,
TiCl4 was the first titanium source to be utilized but it turned out
to not be very convenient because of its hydrolytic instability. We therefore
turned to crystalline complexes of TiCl4, which were much more convenient
to handle. Titanium(II) and -(III) complexes have also been employed as
titanium sources. Reactions of acetylene with titanium sources were carried out
in the present of reducing agents lower the oxidation state of the titanium during
reactions. We experimented with sodium amalgam, sodium and lithium metals and
sodium naphthalenide as reducing agents, and all do effect reduction of Ti(III)
and Ti(IV) compounds.
A major source of acetylide
dianion was acetylene, obtained in pressurized cylinders in acetone solution
and thus requiring purification; we developed a procedure to remove the acetone,
and the purity of the acetylene obtained was assured using NMR spectroscopy. Another
source utilized was sodium acetylide, NaC2H, which was reacted
directly with titanium sources directly or treated with butyl lithium to
produce Na2C2. Note that these compounds also deprotonate
both acetylene and NaC2H
As pointed out previously, report, a large number of experiments was
carried out in attempts to synthesize Ti8C12. The low I.P. and near zero HOMO-LUMO
gap of Ti8C12 suggested that this material would be
obtained as a black, metallic solid which might well be partially oxidized by
traces of water or oxygen to the [Ti8C12]+
cation. We obtained a large number of interesting, insoluble solids which were
characterized by DRIFT spectroscopy, XPS and elemental analyses. We also
analyzed the resulting solutions for metcar cations using electrospray
ionization (ESI) mass spectrometry. Unfortunately, while intriguing observations
were made in some cases, we had difficulties with reproducibility. We do not
know the reasons for the problems, but we suspect that very low yields may in
some cases have rendered the products sensitive to low levels of impurities.
Research during the second year
included more stringent drying of the solvents utilized and a series of
attempts to duplicate some of the more promising reactions previously carried
out. We also extended our previous research with acetylene utilizing Ti(II)
precursors with no reducing agent. It was hoped that disproportionation of e.g.
soluble TiCl2 complexes might give us the mix of oxidation states
that the formation of Ti8C12 requires. Black, air-sensitive,
metallic looking materials were obtained in THF at room temperature, and these
exhibited broad peaks in the DRIFTS spectra at 1300
and 1656 cm-1. These materials turned yellow in air, and thus are
quite different from the metallic substances, reported a year ago from somewhat
similar reactions, which may have been various forms of polyacetylene. ESI MS
studies of the supernatants from the reactions revealed no peaks suggestive of
metcars.
In attempts to prevent the
aggregation of metcars clusters to insoluble materials by stabilizing the Ti8C12
units, some reactions were also carried out under an atmosphere of CO. Previous
gas phase MS studies of the [Ti8C12]+ cation
had shown that it can coordinate several Lewis bases, and we hoped that the
neutral species might be trapped by CO as it formed and be prevented from
aggregation to insoluble materials. Unfortunately no carbonyl stretching bands
were observed in the IR spectra of the solids or of the supernatants.
As a different approach, one which had not
occurred to us previously, we utilized two titanium precursors already in their
desired formal oxidation states, TiCl3(THF)3 and Ti(bipyridal)3,
in a 1:1 molar ratio, with the acetylide salt Li2C2,
formed by reacting acetone-free acetylene with two equivalets of butyl lithium.
This approach avoided actually using a reducing agent, and a reaction carried
out in THF at -20 °C gave a black
precipitate which exhibited bands in the DRIFT spectrum which could be attributed
to a metcar or to bipyridal (possibly coordinated to a metcar as suggested for
CO above). ESI MS experiments on the supernatant showed no interesting peaks
but an XPS spectrum suggested the value of pursuing this line of research
further. We are continuing to pursue this project with funding from a different
source, and we are also utilizing
a literature procedure found very recently for generating Na2C2.
Heating of commercially available NaHC2 under reduced pressure
induces disproportionation to Na2C2 and acetylene, and
the thus formed Na2C2 is being used in parallel with Li2C2
in experiments involving TiCl3(THF)3 and Ti(bipyridal)3.
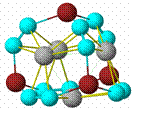 The
original proposal was to develop rational syntheses of metallocarbohedrynes
(met-cars) such as Ti8C12 (pictured). This class of
compounds had previously been synthesized in plasmas and had been studied only
in the gas phase at high temperatures. However, the properties of several
metcars are quite stunning. For instance, Ti8C12 has an
ionization potential between those of sodium and potassium and a near zero HOMO-LUMO
gap, properties most unusual for molecular substances and suggesting metallic
properties and extremely interesting chemistry. This compound is found to
coordinate methane, raising the possibility that it might exhibit interesting
properties as an alkane
functionalization catalyst, while computations suggest that Ti8C12
should be able to bind sufficient hydrogen molecules per cluster that it might
function as a high capacity hydrogen storage system. Synthetic routes to
metcars in the condensed phase are clearly desirable and the original proposal
outlined several possible rational approaches to this end.
The
original proposal was to develop rational syntheses of metallocarbohedrynes
(met-cars) such as Ti8C12 (pictured). This class of
compounds had previously been synthesized in plasmas and had been studied only
in the gas phase at high temperatures. However, the properties of several
metcars are quite stunning. For instance, Ti8C12 has an
ionization potential between those of sodium and potassium and a near zero HOMO-LUMO
gap, properties most unusual for molecular substances and suggesting metallic
properties and extremely interesting chemistry. This compound is found to
coordinate methane, raising the possibility that it might exhibit interesting
properties as an alkane
functionalization catalyst, while computations suggest that Ti8C12
should be able to bind sufficient hydrogen molecules per cluster that it might
function as a high capacity hydrogen storage system. Synthetic routes to
metcars in the condensed phase are clearly desirable and the original proposal
outlined several possible rational approaches to this end.
Copyright © American Chemical Society

