AmericanChemicalSociety.com
Reports: ND7 48942-ND7: Phase Transitions in Mixtures of Micelles and Polyelectrolytes: Electrostatic Assembly of Soft Colloids
Anthony D. Dinsmore, University of Massachusetts (Amherst) and P. L. Dubin, University of Massachusetts (Amherst)
We have
performed experiments to elucidate the mechanisms that drive phase separation
in mixtures of polyelectrolytes and oppositely-charged particles. Mixtures of
this kind are common in industrial settings e.g., in tertiary oil
recovery, in cosmetics, and in materials for drug delivery. Many years of
experiments have shown that these 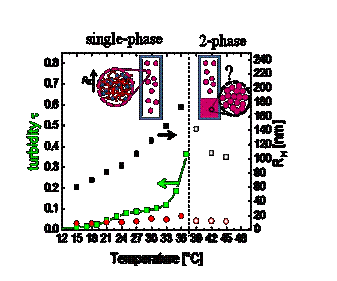 mixtures
can undergo phase separation, often into two coexisting liquid phases. This
process is known as coacervation and is thought to arise principally from
electrostatic and entropic interactions that lead to a complex of positively
and negatively charged molecules and particles. The overall behavior of these
systems depends on many parameters and an overall understanding has so far
eluded researchers.
mixtures
can undergo phase separation, often into two coexisting liquid phases. This
process is known as coacervation and is thought to arise principally from
electrostatic and entropic interactions that lead to a complex of positively
and negatively charged molecules and particles. The overall behavior of these
systems depends on many parameters and an overall understanding has so far
eluded researchers.
The goal of these experiments is to characterize the structures that appear near the phase transitions. Previous reports have shown that very large aggregates (up to 100 nm in size) appear spontaneously in the sample even in the single-phase region. These aggregates are stable in suspension and form reversibly: they are in equilibrium. Here we measure the size (RH) and concentration of these aggregates throughout the phase diagram and ascertain how they depend on polymer molecular weight, sample stoichiometry, and temperature. We seek to establish a connection between the size of these aggregates and the structure of the new phase that emerges during coacervation. We are motivated by the hypothesis that the aggregates can be viewed as effective colloidal particles, and that coacervation may be viewed as phase separation of these “colloidal” particles. By this hypothesis, the coacervate would contain density variations over a size scale similar to the size of aggregates in the coexisting dilute liquid phase.
Our experiments are done with mixtures of a polycation and anionic micelles with tunable surface charge density. Characterization is primarily done using static and dynamic light scattering, neutron scattering (in progress) and optical microscopy. We systematically explore the roles of temperature, concentration of cationic and anionic species, and polymer molecular weight.
An important result is that the size of the aggregates (RH) increases as the phase transition is approached and that the size varies smoothly across the transition. The figure shows a sample in which RH reached 180 nm, which is far larger than the size of an individual micelle or polymer and therefore represents a very large, self-assembled complex. Our results indicate that the growing size and concentration of aggregates play a key role in stimulating the phase transition. Our focus is now turning toward experimental characterization of the structure of the coacervate phase (by microscopy and scattering).
This project has provided rigorous training for our postdoctoral Fellow, a graduate student, and an undergraduate who earned her degree earlier this year. The participants in this project receive interdisciplinary training in a collaboration between faculty in the Physics and Chemistry departments at UMass Amherst.
Copyright © American Chemical Society

