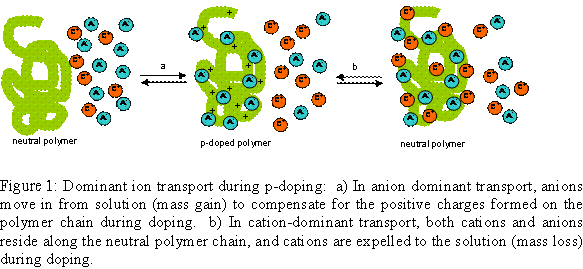AmericanChemicalSociety.com
Reports: UNI10 50120-UNI10: Ionic Liquid Mobility in Electroactive Polymers
Jennifer A. Irvin, Texas State University (San Marcos)
BACKGROUND
Electroactive polymers (EAPs) can generally be switched between two or more stable oxidation and reduction (redox) states. Changes in redox states of EAPs require movement of ions in order to maintain electroneutrality. Ion choice can affect morphology, electrochemical stability, and oxidation and reduction potentials. Ions are usually introduced as a solution of molecular electrolyte, although recently ionic liquids such 1-ethyl-3-methyl-imidazolium bis(trifluoromethanesulfonyl)imide (EMIBTI) have been investigated as electrolytes.1,2 Ionic liquid electrolytes (ILEs) are advantageous due to their wide temperature use window,3 low volatility, and good electrochemical and thermal stability.4
Electrochemical neutralization of p-doped polymers can be anion-dominant (anions are expelled from the polymer film) or cation-dominant (cations diffuse into the polymer film) (Figure 1).5,6 Ion transport processes are best studied using an electrochemical quartz crystal microbalance (EQCM).7,8 In EQCM studies of EAPs, the dominant ion transport process can be determined when the polymer is cycled between the neutral and doped states. An increase in mass during p-doping signifies anion-dominant transport, while a decrease signifies cation-dominant transport. While ion transport processes in p-doping polymers using molecular electrolyte solutions are well-studied, ion transport processes in p-doping polymers using ionic liquid electrolytes have not been thoroughly investigated. Lu and co-workers reported that the process appears to be cation dominant based on visual observation of actuator bending directions during p-doping and re-neutralization.9
Poly(3,4-ethylenedioxythiophene) (PEDOT) is a well-known polythiophene derivative whose electrochemical properties have been thoroughly investigated.10,11,12 When molecular electrolyte solutions are used, anion movement is the dominant ion transport process11 unless the anions are very large.
RESULTS AND DISCUSSION
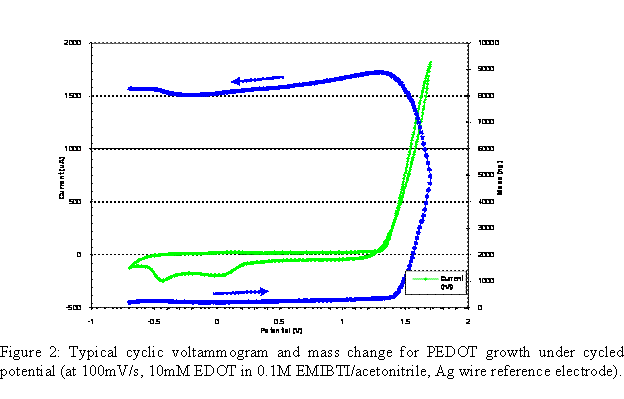
To further explore the ion transport processes of ionic liquid electrolytes in p-doping polymers, we have examined the electropolymerization and switching of PEDOT in the ionic liquid electrolyte EMIBTI. The deposition of PEDOT films was accomplished by electropolymerization of EDOT that had been dissolved in an electrolyte, either 0.1M EMIBTI/acetonitrile or neat EMIBTI. Figure 2 shows that the polymerization of EDOT (0.010M in 0.1M EMIBTI/acetonitrile) is accompanied by increasing mass on the quartz crystal. Onset of monomer oxidation occurs at ca. 1.3V vs. Ag wire. As the newly-deposited polymer is neutralized, a decrease in mass occurs, indicative of anion-dominant transport. A plot of charge passed during electropolymerization versus mass increase is linear, so the PEDOT film is rigid and free of viscoelastic effects, and the Sauerbrey equation applies.13
After polymerization, the polymer, cell, and electrodes were rinsed with monomer-free electrolyte solution, in which the polymer was then cycled at a wide range of scan rates. Current response is directly proportional to scan rate; this indicates that the polymer is electroactive and immobilized on the electrode. All the polymer films reported here show a linear relationship between peak current response and scan rate.
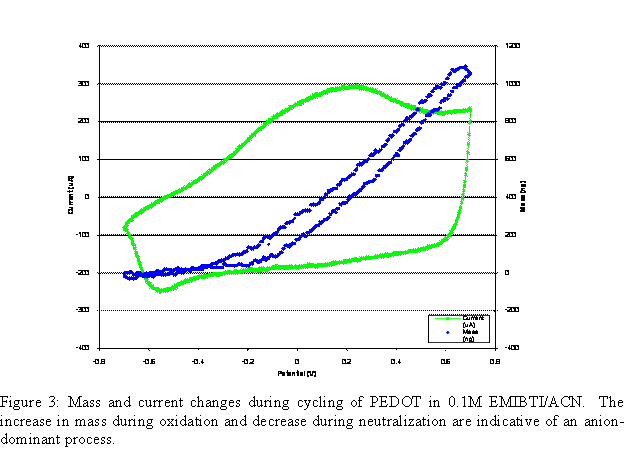
Mass change during cycling of the polymer film can be seen in Figure 3. For 0.1 M EMIBTI/acetonitrile, mass increases during oxidation and decreases during neutralization. This is indicative of an anion-dominant process, typical of p-doping polymers in electrolyte solutions where the anion is relatively small.
PEDOT growth from neat EMIBTI is similar to that of the 0.1M EMIBTI case; the polymerization of EDOT (0.010M in neat EMIBTI) is accompanied by increasing mass on the quartz crystal. Onset of monomer oxidation occurs at ca. 1.25V vs. Ag wire. As the newly-deposited polymer is neutralized, a decrease in mass occurs, which is typically indicative of anion-dominant transport. A plot of charge passed during electropolymerization versus mass increase is linear, so the PEDOT film is rigid and free of viscoelastic effects, and the Sauerbrey equation applies.
After polymerization, the polymer, cell, and electrodes were rinsed with monomer-free electrolyte solution, in which the polymer was then cycled at a wide range of scan rates. The results are similar to those for the 0.1M EMIBTI/ACN example: current response is directly proportional to scan rate, even at higher scan rates.
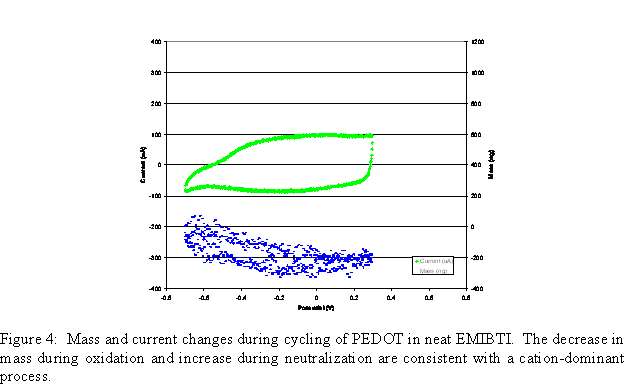
Mass change during cycling of the polymer film can be seen in Figure 4. For the neat EMIBTI case, mass appears to decrease slightly during oxidation and increase during neutralization. This indicates a cation-dominant process, which is unusual for a p-doping polymer with a small anion. Note also that current response of this film is considerably lower than that of the film grown from the 0.1M EMIBTI/ACN solution, indicating that this film is not as electroactive.
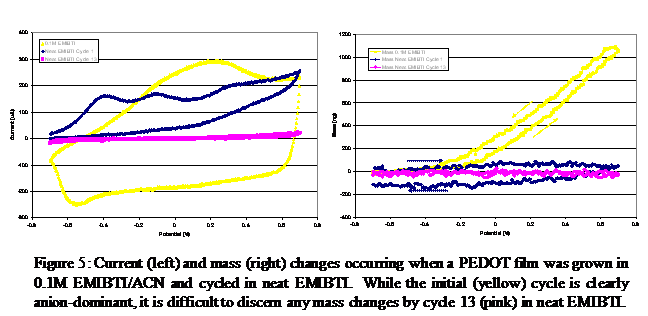
Finally, films grown in 0.1M EMIBTI/ACN were rinsed with neat EMIBTI and then switched in neat EMIBTI (Figure 5). The decrease in current response as trapped acetonitrile is cycled out of the film is remarkable. Eventually, the decrease levels off, and the performance of the film closely resembles that of the film grown from neat EMIBTI. Note also that the ion transport process is clearly anion-dominant in 0.1M EMIBTI/ACN, but no mass change is discernable after 13 cycles in neat EMIBTI.
CONCLUSIONS
While p-doping of PEDOT in a solution of EMIBTI was, as with other molecular electrolyte solutions, found to be anion-dominant, p-doping of PEDOT in neat EMIBTI was found to be cation-dominant. This work was presented at the International Conference on the Science and Technology of Synthetic Metals in Kyoto, Japan in July 2010.
REFERENCES
1 Pringle, J. M., et al., Polymer, 46, 2047, 2005.
2 Stenger-Smith, J.D., et al., J. Electrochem. Soc., 149(8), A973, 2002.
3 Stenger-Smith, J.D., et al., J. Electrochem. Soc., 157(3), A298, 2010.
4 Stenger-Smith, J. D. and Irvin, J. A., Material Matters, 4(3), 103-105, 2010.
5 Ding, J. et al., Chem. Mater., 15, 2392, 2003.
6 Yang, H. and Kwak, J., J. Phys. Chem. B, 102, 1982, 1988.
7 Niu, L., Kvarnstrom, C., and Ivaska, A., J. Electroanal. Chem., 569, 151, 2004.
8 Krivan, E., Visy, C. and Kankare, J., Electrochim. Acta, 50, 1247, 2005.
9 Lu, W. et al., Science, 297, 983, 2002.
10 Groenendaal, L. et al., Adv. Mater., 12(7), 481-494, 2000.
11 Bund, A. and Neudeck, S., J. Phys. Chem. B, 108, 17845-17850, 2004.
12 Yang, N. and Zoski, C. G., Langmuir, 22, 10338-10347, 2006.
13 Naoi, K., Lien, M., and Smyrl, W. H., J. Electrochem. Soc., 138(2), 440-445, 1991.
Copyright © American Chemical Society