AmericanChemicalSociety.com
Reports: ND10 49260-ND10: Organic and Inorganic Architectures for Solution-Processable, Solar Photovoltaics
Cherie R. Kagan, PhD, University of Pennsylvania
We are tailoring the interface and architecture of bulk heterojunction blends and bilayers of organic semiconductors and functionalized fullerenes or inorganic nanocrystals (NCs) or nanowires (NWs). Using the solution-based precursor route to pentacene [Fig. 1], we are working to engineer the hybrid architecture by modifying the precursor functionality and the electronic structure of the hybrid by combining organic and II-VI and IV-VI NC and NW building blocks. We have also constructed new measurement capabilities to study the physics and performance of these hybrid materials as the active layers in solar cells.
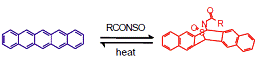
| Fig. 1 Pentacene is insoluble in common solvents. Diels-Alder chemistry provides a soluble precursor that is deposited from solution in hybrid solar cells and thermally retro-converted to pentacene. |
Soluble pentacene precursors with varying side chains were synthesized via a Diels-Alder reaction with the meso positions of the pentacene molecule and N-sulfinyl moieties [Fig. 1]. The reaction was catalyzed using catalysts such as Re, Pd, and Sn allowing for optimization of reaction conditions. The N-sulfinylacetamidopentacene was synthesized routinely and used in spectroscopic studies and in the fabrication of organic-inorganic solar cells. PbSe NCs and NWs were synthesized by wet chemical methods. The pentacene precursors and semiconductor nanostructures are soluble/dispersable, allowing organic-inorganic bulk heterojunctions to be deposited from solution. The pentacene precursor is thermally converted to pentacene while evolving the N-sulfinyl small molecule [Fig. 1].
Synthetic efforts were devoted towards the design of a new, soluble pentacene precursor with a pendant phosphonic acid. The N-sulfinyl diethylphosphoramidate adduct is a known molecule. Initial synthesis from commercially available diethyl phosphoramidate and thionyl chloride in refluxing benzene had side products, difficult to remove via distillation. Adding the step of coupling a trimethylsilyl group to the amine was used to form an amide. The amide was then reacted with thionyl chloride at room temperature in benzene producing the desired N-sulfinyl diethylphosphoramidate in nearly quantitative yield. With the N-sulfinyl adduct, Re catalyzed Diels-Alder reactions were attempted with little to no yield. Attempts then focused on using fuming tin(IV) tetrachloride as the catalyst. It is clear from the reaction mixture that the reaction proceeds quantitatively as pentacene is consumed; the reaction changes color from deep blue to a clear-turbid solution. Aliquouts of the reaction mixture confirm the presence of the adduct by thermally converting the clear-turbid solution to a blue film. However, the Diels-Alder adduct appears very unstable, rapidly undergoing retro-Diels-Alder upon addition of base or water to break apart the tin chelates. Reaction conditions utilizing several different Lewis acid catalysts (TiCl4, BF3, AlCl3, InCl3) were explored, however, with similar results. It is unclear if the instability is related to steric or energetic origins. Attempts to better understand this phenomenon to develop alternative routes towards a phosphonic acid pentacene precursor using similar reactions were performed starting with N-sulfinyl benzamide. Currently, catalysts are being investigated for the reaction. If successful, the phosphonic acid group can be functionalized onto the phenyl ring prior to forming the sulfinyl group for the Diels Alder reaction.
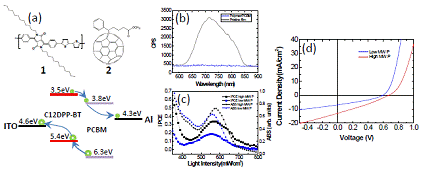
We have also recently synthesized and characterized the polymer C12DPP-BT 1, which brings together electron deficient C12DPP and electron rich bithiophene, in low and high molecular weights [Fig. 2(a)]. The synthesis of 1 was obtained by the reaction of 5,5′-bis(trimethylstannyl)-2,2′-bithiophene with 1 equiv of 3,6-bis(4-bromophenyl)-2,5-didodecylpyrrolo[3,4-c]pyrrole-1,4-dione by Stille cross coupling in the presence of a catalytic amount of Pd(PPh3)4 in toluene. A shiny light brown solid was acquired which has lower Mn of 5,833 g/mol (PDI = 1.76). The catalyst Pd2(dba)3/P(o-tolyl)3 was adopted and much higher molecular weight of polymer (Mn of 12,365 g/mol, PDI = 1.43) was yielded.
| Fig. 2 (a) Chemical structures of the polymer C12DPP-BT 1 and PCBM 2. The solar cell electronic structure is derived from cyclic voltammetry measurements and reported metal work-functions. Photoluminescence quenching (b), internal photoconversion efficiency and absorption (c), and I-V measurements of the hybrid blend solar cells (d).
|
We co-dispersed 1 with 2 to spin-coat bulk heterojunction blends. Using photoluminescence, we have shown that blending 1 with 2, completely quenches the C12DPP-BT emission [Fig. 2(b)], consistent with charge transfer across the type-II organic-inorganic heterointerface. UV-visible absorption measurements show an increased absorbance and red-shift of the high molecular weight 1, consistent with increased inter-chain interaction (film thickness is the same for both samples). Internal photoconversion efficiency measurements (IPCE) [Fig. 2(c)] show absorption of excitations in both the organic semiconductor and PCBM give rise to charge generation. As observed from the IPCE measurements, the high molecular weight polymer more effectively transfers and transports charge as seen in the I-V characteristics [Fig. 2(d)], where the higher molecular weight polymer has an increased short circuit current and open circuit voltage. The power conversion efficiency increases from 1.6% for the low molecular weight polymer to 2.6% for the high molecular weight polymer. This derivative shows amongst the higher solar cell efficiencies in comparison to similar C12DPP polymers.
| Fig. 3 Optical micrographs and photocurrent maps of C12DPP-BT:PCBM blend (a) with and (b) without diiodoctane additive
|
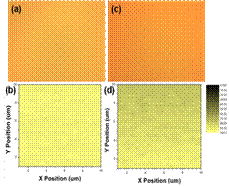
We also have built new measurement capabilities to study the physical phenomena and device characteristics of organic-inorganic materials and their solar cells. We modified a flourimeter and constructed sample cells to simultaneously measure the internal photoconversion efficiency and excitation-dependent photoluminescence of solar photovoltaics, extending to long wavelengths (~2.5 µm). The internal conversion efficiency, for example, is shown in Fig. 2(c). We have also built a confocal optical microscope to simultaneously spatially resolve the photoconductivity and photoluminescence from solar materials and devices. We designed and constructed electronic boards and masks to pattern substrates to carry out spatially resolved photoconductivity mapping measurements through the optical microscope. Fig. 3 shows maps of the short circuit current of C12DPP-BT/PCBM hybrid solar cells with an without the additive diiodoctane, which has been reported to increase solar cell efficiency affecting phase separation in hybrids. The maps show that the additive provides a higher and consistent current in comparison to the same active material without the additive, consistent with the higher measured power conversion efficiency.
Copyright © American Chemical Society