AmericanChemicalSociety.com
Reports: UNI10 49468-UNI10: Methanol Synthesis from CO2 and H2 Utilizing Bimetallic Nanoparticle Catalysts
John D. Gilbertson, PhD, Western Washington University
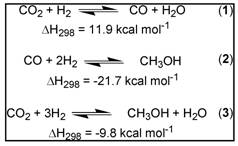
Dwindling fossil fuel supplies and global warming from rising carbon emissions are the potential cause of economic and environmental crises of global proportion. During the past century, atmospheric CO2 levels have increased significantly, and global temperatures are predicted to subsequently rise ~2-4 °C by the end of this century. A viable solution to these quandaries is the utilization of CO2 as a primary C1 source, which could help stave off potential economic and environmental catastrophes by converting CO2 into a useable commodity such as methanol. The formation of methanol from CO2 and syngas proceeds through the equilibria in equations 1-3 and is exothermic. Optimum reaction conditions typically require low temperatures and increased pressure.
Funded by a two year
American Chemical Society Petroleum Research Fund (Type UNI) Grant (May 2009 –
May 2011), the synthesis, characterization, and evaluation of Cu-based
bimetallic nanoparticle catalysts for carbon dioxide
hydrogenation (from CO2 and H2) is being
investigated. The catalysts are prepared
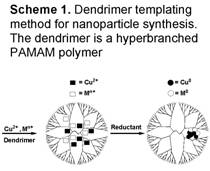
via the dendrimer templating
method (Scheme 1) which allows us to
exercise careful control over the particle size, composition, and morphology of
the catalysts. The dendrimer-encapsulated
nanoparticles are deposited onto the appropriate
support before removing the dendrimer template
thermally to produce active catalysts. Typically
the preparation of bimetallic particles of uniform size, composition, and
morphology is exceedingly difficult and our approach allows us to overcome the major limitations that are present in
the development of current systems; such as metal segregation, agglomeration,
and wide particle size distributions. The dendrimer templating method allows us such control and we are currently
using it to prepare Cu-M bimetallic nanoparticles (M
= Au, Pd, Pt, and Ni initially) deposited onto different oxide supports (ZnO, SiO2, and Al2O3). We have chosen Au, due to its well behaved
nature which allows us to optimize our synthetic techniques. Pd, Pt, and Ni were chosen due to their
propensity for hydrogenation activity. To date we have successfully prepared a series of Cu/ZnO catalysts ranging from 10 - 50 wt% Cu based on the co-precipitation method. We have also prepared a series of Cu-based
mono- and bimetallic nanoparticle catalysts which
include Cu25/ZnO, Ni25Cu25/ZnO, Cu25/SiO2,
and Cu25Au25/SiO2 (all 2.5 wt % metal) via the dendrimer templating method. (The n in Cun represents the number of atoms in the nanoparticles after synthesis and before thermal activation
to remove the dendrimer.) Included in the preparation of these
catalysts are the proper dendrimer removal conditions
(calcination in an O2/N2
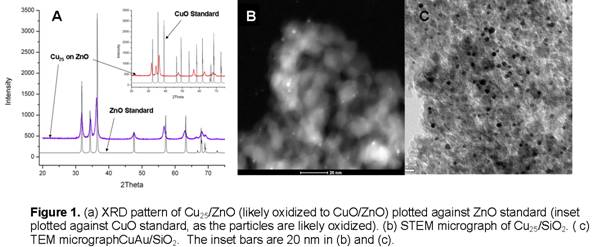
mixture, followed by H2/N2). A representative XRD pattern of the catalysts
are presented in Figure 1a. The XRD
pattern is indicative of particle sizes that lie below the size limit
accessible by XRD (< 5 nm). All of
our catalysts synthesized via the dendrimer-templating
method to date yield no information in their respective XRD patterns due to
small particle size. Fig 1b confirms
that in the case of Cu25/SiO2 we are indeed producing
particles on the order of 2 nm (particle size distribution of 2.44 +/- nm). Figure 1c shows a TEM micrograph of CuAu/SiO2. We have recently finished the construction of a high
pressure single pass plug flow methanol synthesis microreactor. This allows us to vary the pressure of the H2/CO2
3:1 feed from atmospheric pressure up to 20 MPa. We are currently calibrating the reaction and
obtaining the optimal temperatures and pressures with which to perform our
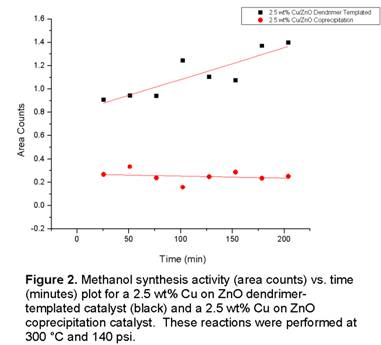
activity measurements on the synthesized catalysts. Our initial data (Figure 2) shows that the
Cu/ZnO catalysts synthesized from the dendrimer-templating method are more active for the production
of methanol from CO2/H2 than a control Cu/ZnO catalyst synthesized by the co-precipitation method.
Copyright © American Chemical Society