AmericanChemicalSociety.com
Reports: DNI5 49261-DNI5: Surface Organometallic Chemistry for Improved Performance and Understanding of Hydrodenitrogenation Catalysis
Justin M. Notestein, PhD, Northwestern University
Hydrotreating is the essential process of removing N and S from crude oil and other fuels. As the U.S. moves to crudes with higher heteroatom content, there is a significant need for fundamental understanding of hydrotreating catalyst design and mechanisms. Hydrodenitrogenation (HDN) has been studied less and is particularly energy inefficient. Therefore, research has been undertaken to synthesize and study model HDN catalysts using Ta and Nb surface organometallic chemistry. Ta and Nb are chosen due to intriguing C-C, N-N, C-N cleavage activity by surface and soluble organometallic complexes. On surfaces, these isolated cations are also expected to only begrudgingly saturate aromatic rings, which is a challenge to the H2 efficiency of existing processes. In addition to specific insights into HDN, new syntheses are developed for supported catalysts, particularly group 4/5 supported catalysts.
To date, several new supported Ta catalysts have been synthesized, characterized, and tested in alkene epoxidation (extensive) and alkyne hydroamination and HDN (preliminary). A student has not yet been placed full-time of this project; the work described here is partially supported by ACS PRF but primarily supported by a self-funded postdoctoral fellow.
SYNTHESIS AND CATALYTIC TESTING
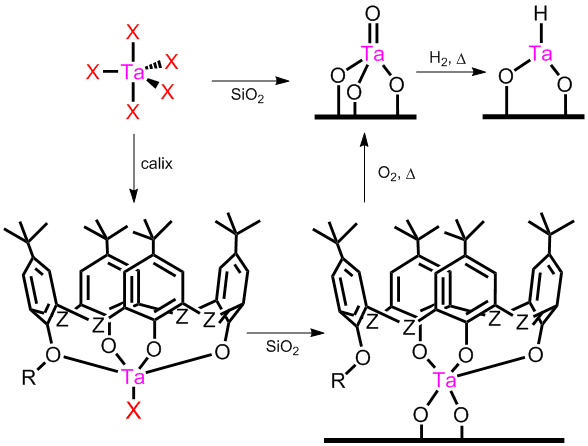
Following the proposal, organometallic Ta(Bz)5, TaCl5, Cp*TaCl4, and Ta(N(CH3)2)5 have been grafted to silica under in Ar-filled gloveboxes. The surface reaction is assumed to proceed by alcoholysis or simple ligand exchange. Calixarene-Ta complexes have also been grafted to silica by adding stoichiometric calixarene in toluene to TaCl5 or Cp*TaCl4, refluxed, and then previously dried silica is added to the same vessel to support the complex. (Figure 1) Intermediate complexes are not isolated because reactions are known to be high yielding. Ligands tert-butylcalix[4]arene, dimethoxy-tert-butylcalix[4]arene, tert-butylcalix[6]arene, and tert-butylthiacalix[4]arene have been used. Synthesis details are available in two recent publications that cite the support of ACS PRF.
FIGURE 1. Synthesis of supported Ta catalysts.
These catalysts have been tested for alkene epoxidation with H2O2. This requires highly isolated surface cations, which is also desired for our HDN catalysts, making this a relevant probe. Ta-SiO2 has been minimally studied as a solid epoxidation catalyst, but our work now shows it is active and exceptionally selective. The calixarene ligand, weakly dependent on the specific type, enforces Ta site-isolation, ensuring stable rates and high selectivity even at high catalyst loading, characteristic of ‘single-site' catalysts. The reader is directed to two recent publications that cite the support of ACS PRF for more details.
Directly in support of this proposal, these catalysts have been tested in C-N bond breaking and forming reactions, although results are still preliminary. Alkyne hydroamination with aniline and alkyne hydration via a hydroamination intermediate have been carried out with heat-treated and as-synthesized supported Ta(V) catalysts. Activity is seen in these oxidative reactions, which require isolated sites and the formation of Ta=N intermediates, both of which are necessary for HDN. Quantitative results are not available because silica promotes side reactions that make product characterization challenging.
Calixarene-Ta-SiO2 catalysts have also been calcined at 550°C, reduced at 350°C, and used in initial testing of quinoline hydrodenitrogenation with 210 psig H2. Reduction and reaction conditions are given in Table 1. After H2 treatment, the catalysts darken substantially, consistent with reduction of isolated, d0 Ta(V) to d2 Ta(III) or Ta nanoparticles. Initial results for these catalysts are compared to Pd/Al2O3.
TABLE 1. Catalysts and conditions for HDN. Tentative product assignments are in the text. Products are given as % of observed. Mass balance has not been verified.
Catalyst | Conditions | Conversion | Product % (A, B, C, D) | |||
Pd/Al2O3 (25 mg) | 275°C, 2h | 87% | 40 | 8 | 10 | 41 |
Pd/Al2O3 (5 mg) | 275°C, 2h | 63% | 3 | 0 | 6 | 90 |
Ta-SiO2 | 275°C, 2h | 38% | 0 | 0 | 9 | 90 |
Ta-SiO2 | 350°C, 2h | 42% | 0 | 0 | 10 | 90 |
Ta-SiO2 | 350°C, 20h | 58% | 0 | 0 | 12 | 87 |
At similar conversions (from 100 mg of the highly dispersed Ta-SiO2 or 5 mg of the precious metal Pd/Al2O3 catalyst), product distributions are very similar, suggesting that the observed products follow typical reaction networks, with A and B as propylcyclohexylamine and decahydroquinoline, while C and D are tetrahydroquinolines, based on GC retention times. We will verify product assignments and look for propylaniline at higher conversions.
CHARACTERIZATION.
Ta(V) catalysts have been characterized by TGA for decomposition of calixarene-Ta-SiO2 and diffuse reflectance UV-visible spectroscopy (DRUV), which conclusively identified highly dispersed Ta centers, and in the case of calixarene-containing catalysts, direct ligand-metal-surface connectivity. XAS at the Advanced Photon Source confirms the presence of highly isolated Ta(V), but fine structure characterization has not yet been possible. SS 13C CP/MAS NMR and DRIFTS confirm the same connection and loss of the ligand after calcination. The reader is referred to two recent publications. TPR shows reduction between 350 and 500ºC. DRUV and XAS under reducing conditions are planned for the near future to identify active species, oxidation states, and the extent of aggregation, if any, under reaction conditions.
Copyright © American Chemical Society