AmericanChemicalSociety.com
Reports: B10 45004-B10: Investigating the Chemistry of Boranes with Single-Walled Carbon Nanotubes Using FTIR and Raman Spectroscopies
Mark Ellison, Ursinus College
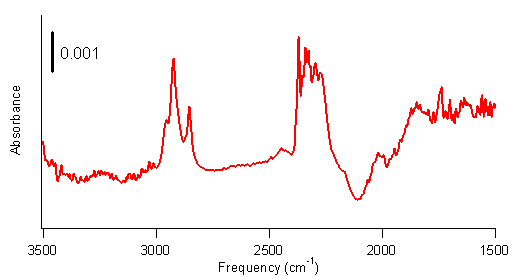
Work in the first year of the grant found evidence that borane complexes did not react with single-walled carbon nanotubes (SWCNTs) at 0°C or at room temperature. However, research in fall 2007 found that, when irradiated with light from a mercury lamp, SWCNTs do appear to react with borane-tetrahydrofuran. Continuation of this work in summer 2008 found that SWCNTs appear to react with borane-dimethyl sulfide but not with borane-triethylamine or borane-tert-butylamine when irradiated with light from a mercury lamp. These results suggest that the more reactive borane complexes will react with SWCNTs when irradiated with light from a mercury lamp, but the more stable borane complexes will not. The reaction appears to be a hydroboration reaction, which attaches a –BH2 group and a –H group to the nanotubes. Efforts to isolate these intermediates were difficult, presumably because of the reactivity of the –BH2 group. Nonetheless, two of the student researchers, through careful experimentation, did reproducibly obtain infrared spectra of SWCNTs with peaks at 2300 cm–1 and 2950 cm–1 attributed to B-H stretches and C-H stretches, respectively, as shown in Figure 1.
Figure 1. FTIR spectrum of SWCNTs reacted with
borane-tetrahydrofuran complex under irradiation from a mercury lamp. Peaks at 2300 cm–1 are consistent
with B–H stretches, and peaks at 2950 cm–1 are consistent with C–H
stretches. Work in spring 2008 determined that the reaction is
initiated by light of wavelength less than 300 nm. This suggests that the SWCNTs are absorbing
the ultraviolet light to undergo a p→ p* transition. This could interrupt the aromaticity of the
nanotubes' structure, increasing their reactivity. Computational studies indicated that the
hydroboration reaction is endoergonic, suggesting that it is likely not
spontaneous without some energy input.
Experiments with optical filters have yielded some evidence for the photochemical
reaction mechanism.
Three optical filters were used. The first, U330, transmits light from the UV
to the edge of the visible range, or from about 250 to 400 nm. The second, U360, transmits light from the
mid-UV to the visible range, or from about 300 to 400 nm. The third, B440, transmits light from the
near edge of the visible range to the middle of that range, or from about 380
to 500 nm. These three filters cover the
UV to the visible in a nearly mutually exclusive manner, allowing us to
ascertain if one particular wavelength region is responsible for exciting the
reactants and leading to reaction. FTIR
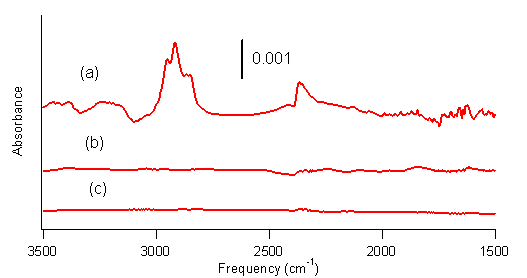
spectra of SWCNTs irradiated with light that passed through one of these three
filters and reacted with borane-tetrahydrofuran are shown in Figure 2. Figure 2a shows SWCNTs from a reaction
mixture irradiated with light that passed through U330. Figure 2b shows SWCNTs from a reaction mixture
irradiated with light that passed through UG360. Figure 2c shows SWCNTs from a reaction
mixture irradiated with light that passed through B440
Figure 2. FTIR spectra of SWCNTs reacted with BH3–THF
for 6 h under mercury lamp irradiation with light passing through (a) filter
U330 (b) filter U360 and (c) filter B440. These results clearly indicate that light in the range of
250-300 nm is necessary to drive the hydroboration reaction. The Hg lamp has a major peak at 254 nm, which
overlaps with the broad UV absorption of SWCNTs at lmax=260 nm.
This strongly suggests that the photochemical hydroboration reaction
proceeds through an electronic excitation of the SWCNTs. An SWCNT that undergoes a p→ p*
transition would be reactive toward nearby molecules, resulting in this case in
a hydroboration reaction.
To verify that a hydroboration reaction had taken place,
SWCNTs reacted with borane-tetrahydrofuran under ultraviolet irradiation were
then reacted with a sodium hydroxide/hydrogen peroxide mixture. These conditions are those of a classic
hydroboration-oxidation reaction, in which borane adds across a double bond to
form –BH2 and –H groups, and the –BH2 group is
subsequently oxidized to –OH. Infrared
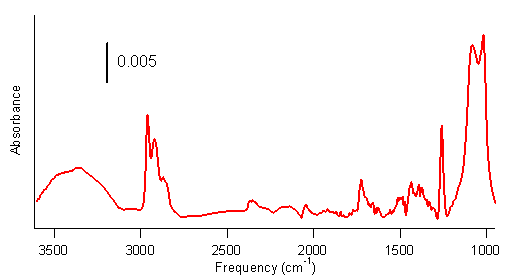
spectra of the SWCNTs subjected to these steps, such as the one shown in Figure
3, show numerous peaks that are consistent with our hypothesis: O–H stretches
at 3360 cm–1, C–H stretching peaks at 2900 cm–1, C–O
stretches at 1090 and 1260 cm–1, and a C–H bend at 1400 cm–1. This strongly suggests that the SWCNTs are
undergoing a photo-initiated hydroboration/oxidation reaction.
Figure 3.
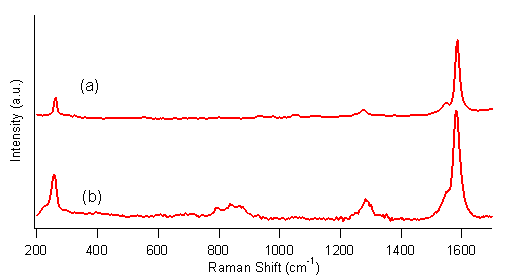
FTIR spectrum of hydroborated–oxidized SWCNTs. The Raman spectrum of the purified SWCNTs, shown in Figure 4a,
shows the radial breathing band at ~250 cm–1
and the G band at ~1580 cm–1,
both of which are characteristic of SWCNTs.
The Raman spectrum of hydroborated-oxidized SWCNTs, Figure 4b, shows
these features and an increase in the D band at ~1280 cm–1, which indicates
functionalization of the SWCNTs. The
ratio of D band to G band peak areas of 0.15 is consistent with moderate
functionalization of the SWCNTs.
Figure 4.
Raman spectrum of hydroborated–oxidized SWCNTs. This work is one of just a few reports of photochemical reactions
involving SWCNTs. It is also, to our
knowledge, the first report of the reaction of borane compounds with SWCNTs. Six undergraduate students contributed to
this research, and it has been published in the Journal of Physical Chemistry C.
This brings the initial part of this research to a successful
conclusion, while opening the door for the continued exploration of the
reaction of boron-containing compounds with SWCNTs. This grant has allowed me to continue to
develop as a researcher and has put my research program on solid footing. I am now able to attract about five
undergraduate students per semester and two undergraduate students per summer
to engage in research. This research has
directly supported six undergraduate students and indirectly supported eight
more. Many of these students presented
posters on their research at regional or national conferences. Six of the students who worked on this
research are co-authors on the recently accepted journal article. I thank the ACS PRF for their support to make
this success possible.
Copyright © American Chemical Society