AmericanChemicalSociety.com
Reports: AC9 45927-AC9: Measurement of Molecular and Thermal Diffusion Coeffcients in Model Petroleum Fluids
Abbas Firoozabadi, Yale University
In the following, we will present the modeling work, and then discuss the measurements.
Modeling of Fickian diffusion coefficients of CO2-water mixtures
The increase of CO2concentration in the atmosphere is believed to affect global warming. Various options are being examined for storage of CO2 from major sources. CO2 storage in geological formations is perhaps the most attractive and practical option (Firoozabadi and Cheng, AIChE J., 2010). CO2 injection in oil reservoirs for the purpose of improved recovery is also a partial short-term solution.
The effectiveness of CO2 storage in saline geological formations, and CO2-improved oil recovery depend on the rate of dissolution of CO2 in the brine and in the oil (Hoteit and Firoozabadi, SPE J., 2009; Firoozabadi and Cheng, AIChE J., 2010). The rate of dissolution depends strongly on diffusion flux and consequently on diffusion coefficients. While there are limited data for CO2-hydrocarbons over a wide temperature and pressure range, this is not the case for CO2-water. Most of the data for diffusion of CO2 in water are at temperatures less than 90°C. There are few data points for diffusion coefficients of H2O-CO2 system at pressures encountered at CO2 sequestration in saline aquifers.
We have developed a simple model that accurately describes the infinite-dilution diffusion coefficients for CO2 in water at both dilution limits. We also estimate the composition-based Fickian diffusion coefficients in CO2-rich and water-rich mixtures. We account for the polar nature of water molecules and polarizability of CO2 molecules. Water molecules have a large permanent dipole moment that causes formation of aggregates of its molecules. CO2 has no net dipole moment, but due to its polarizability, it can have induced in the presence of an electric field. When a water molecule approaches a CO2 molecule, a dipole-induced-dipole interaction is produced. The result is the formation of water-CO2 clusters.
Our unified approach for infinite-dilution diffusion coefficients of CO2-H2O mixtures is based in part on the corresponding state principle. We take into account the temperature effect in the total dipole moment of water and the induced-dipole moment of CO2, along with other thermodynamic variables. A single expression is found to describe diffusion coefficients of water infinitely diluted in CO2 as well as CO2 infinitely diluted in water as function of temperature, pressure, molecular mass, dipole moment, molar density and viscosity.
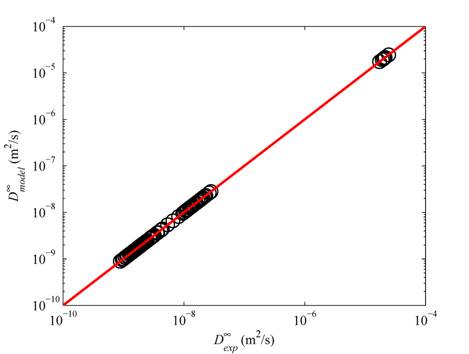
Figure 1 shows comparison of our model results with experiments based on 180 data points for 273 K ≤ T ≤ 368 K and 0.1 ≤ P ≤ 39.2 MPa and 8.9 × 10-10 ≤ D∞ ≤ 2.45 × 10-5 cm2/s.
The model is then used to predict infinite dilution diffusion coefficients outside the range of data used for CO2 -H2O, and for propane-H2O, pentane-H2O, and H2S-H2O. The predictions are in agreement with data.
We have also used the irreversible thermodynamic approach (Ghorayeb and Firoozabadi, AIChE J., 2000) to predict the effect of concentration on diffusion coefficients of H2O-CO2 mixtures.
Figure 1.
Diffusion coefficients of CO2 and water at infinite dilution (D∞)
Experimental Measurements
of Thermal Diffusion and the Soret Effect Using a
Deflected Laser Beam Thermal diffusion arises when a
temperature gradient is imposed across a material, and can cause either mixing
or unmixing of the components. Measurements are available in the literature
for the thermal diffusion of liquid mixtures and large solute systems. However, unanswered questions remain about
the underlying physical phenomena, including the dependence on solvent
interactions and the molecular size and shape of the components. In practical applications, thermal diffusion
can facilitate separations and provide sample characterization, as seen in
thermal field flow fractionation for polymeric characterization (Weigand, J. Phys. Cond. Matt., 2004).
At the same time, measurements of
the diffusion constant provide a basis for theoretical investigation of real
mixtures. Thermal diffusion can be an
important aspect of reservoir modeling and simulations.
Our setup to measure thermal
diffusion consists of a temperature controlled cell through which we pass a
laser beam. We impose a temperature
gradient across the sample and monitor the resulting change in concentration by
recording the deflection of the laser beam as a function of time. Both thermal and molecular diffusion
coefficients can be extracted from the deflection of the laser beam using
established theory (Haugen and Firoozabadi, J. Phys Chem. B, 2006; Zhang,
Briggs,Gammon, Sengers, J. Chem. Phys.,1996).
This past year we have rebuilt
our experimental set-up and improved both the stability of the laser line and
the closed loop temperature control system.
This set-up consists of two copper plates sandwiching a quartz glass
ring. The temperature on the copper
plates is controlled through an electric circuit consisting of power supplies
directing current through thermofoil heaters on each
plate. The setup is controlled in a
close loop fashion through PID software run through LabView,
which allows for all aspects of the experiment to be monitored and controlled
simultaneously. The implementation of
this closed-loop computer control system has resulted in tight temperature
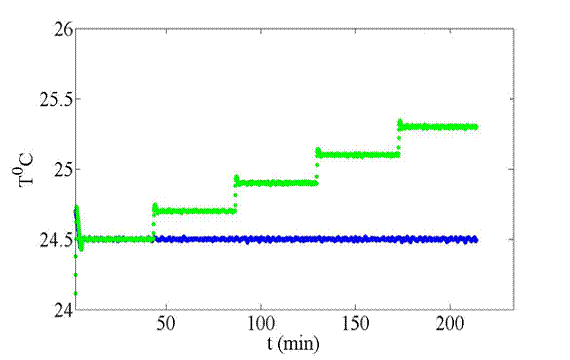
control on the copper plates. Figure 2
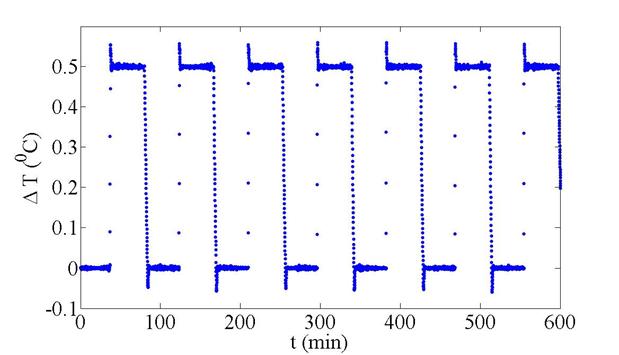
shows the PID-controlled temperature on the top and bottom plates. Figure 3 shows the imposed temperature
difference, stable to within 2.3 mK,
which can be turned on and off many times in a row, to allow for a more
thorough assessment of the experimental measurements.
Testing demonstrates that the setup
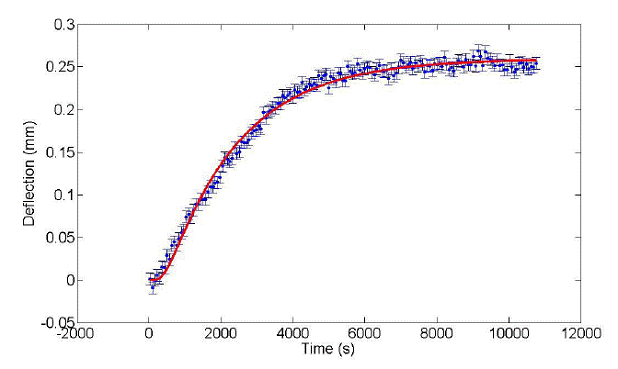
is working properly. Measurements of an equimolar mixture of hexane and toluene give a Soret coefficient of ST =
4.6x10-3 K-1, which paramaterizes
the fit shown in Figure 4. This value is within 5% of other laser-beam
deflection measurements on the same system.
During the coming months we intend to extend measurements using this
setup to novel systems.
Figure 2: The temperature on the
top plate is shown in green, and the bottom plate in
blue, controlled by thermofoil heaters and a PID
loop, for approximately 3.5 hours.
Figure 3: The temperature gradient between the two
plates can be stably turned on and off for many cycles at a time.
Figure
4: Laser beam deflection in an equimolar mixture of hexane and toluene, given
an imposed temperature gradient of 0.5 °C.
Copyright © American Chemical Society