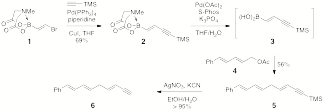AmericanChemicalSociety.com
Reports: GB1 47873-GB1: Rapid, Controlled Assembly of Polyenes for Studying Pericyclic Reaction Cascades
David A. Vosburg, Harvey Mudd College
In collaboration with my theoretical chemist colleague at HMC Robert Cave, we have calculated ground state and transition state energies for the endiandric acid pericyclic reaction cascade using density functional theory methods. We have focused on estimating the relative energetics of endiandric acids B, C, F, and G and the transition states for F to B and G to C (Scheme 1). We have found that the activation energy for the endiandric acid F to B Diels-Alder reaction is slightly lower than that for G to C (for R = CO2H). Consideration of solvent does not significantly affect this trend, but replacing the carboxylic acid with a trifluoromethyl ester or aldehyde results in a lower transition state to form C (Table 1). The corresponding methyl ester and methyl thioester are similar to the carboxylic acid, while replacing the carboxylic acid with a primary alcohol strongly favors the formation of B. The series of calculations we have performed indicates that these systems are tractable to theoretical analysis and that we can make interesting predictions that have the potential to guide synthetic efforts. We are currently preparing a manuscript for the publication of these results.
Scheme 1. Reactions of endiandric acids and derivatives used in computational
studies (see Table 1). Table 1. Calculated relative energetics (kcal/mol) for the activation
energy difference between the F to B and G to
C transition
states for a series of endiandric acid derivatives (varying in the R
substituent in Scheme 1) using B3LYP/6-31G(d).
R = CH2OH CO2CH3 CO2H C(=O)SCH3 CHO CO2CF3 Ea(F to B) - Ea(G to C) = -3.1 -0.9 -0.5 0.0 0.1 1.4 Favored product: B B B – C C Our
recent synthetic studies have focused on commercial, bifunctional alkene 1 as the starting point for several
polyene cascade precursors. Bromoboronate 1 can be reliably converted to enyne 2 under Sonogashira conditions in a
glovebox or a microwave. This enyne can be deprotected in situ and immediately cross-coupled with
a variety of allylic electrophiles, including dienyl acetate 4. This has enabled us to rapidly
assemble trienyne 6 in three steps and 37% yield from 1 (Scheme 2), compared to nine steps
and 15% yield (Nicolaou, et al., J. Am. Chem. Soc. 1982, 104, 5558). Scheme 2. Preliminary results towards the
biomimetic syntheses of endiandric acids A-G. We
are currently optimizing these Suzuki-Miyaura couplings and exploring multiple options
for the remaining steps in the synthesis, including the preparation of (Z)-alkenes for cross-coupling
reactions to trigger the pericyclic cascades. We will continue to annually
report our progress in poster presentations at spring ACS national conferences
and hope to complete the synthesis and publish our results in the near future.
My undergraduate students have done remarkable work on this project, most
recently Andrew Chung '10, Laura Poindexter '11, Brigid Poling '11, and Zara
Seibel '11. In
summary, we have obtained enough computational data for a theoretical
manuscript on the cascade and are close to completing the synthesis of
endiandric acids A-G. We will then be able to move on to test our theoretical
predictions and to synthesize biologically active members of this class of
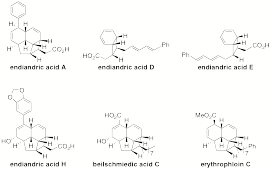
natural products such as endiandric acid H (anti-inflammatory), beilschmiedic
acid C (antibiotic), and erythrophloin C (antitubercular). This work supported
by the ACS-PRF grant has further enabled the submission of a NSF CAREER grant
that was submitted in July 2009 and an NSF RUI grant that was submitted in July
2010. Figure
1. Structures of other
endiandric acids and natural derivatives, including the bioactive compounds
endiandric acid H, beilschmiedic acid C, and erythrophloin C.
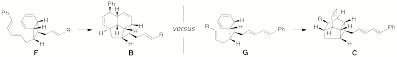
Copyright © American Chemical Society