AmericanChemicalSociety.com
Reports: B6 47262-B6: Examining Hydrodynamic and Solubilization Properties of Micelles Formed by Chiral Amphiphiles with NMR
David S. Rovnyak, Bucknell University and Timothy G. Strein, Bucknell University
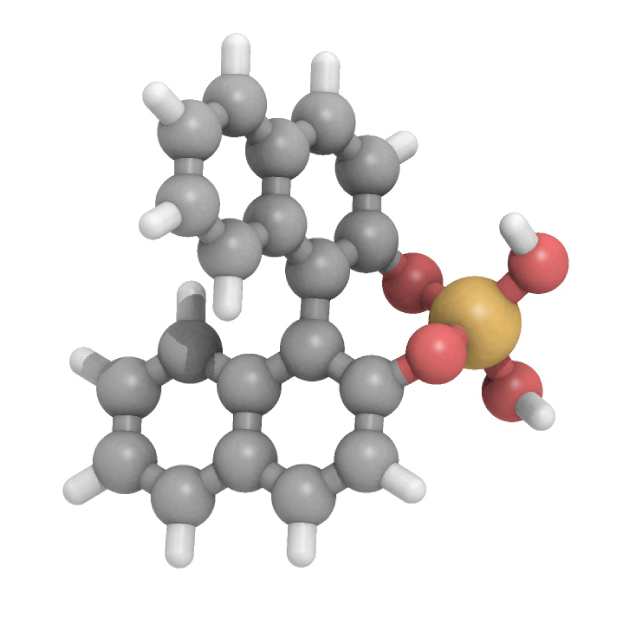
Our efforts in the past year have emphasized NMR diffusometry to characterize how bile salt micelles differentially solubilize the atropisomers R,S-BNDHP. Below is an illustration of S-BNDHP:
Figure
1. S-BNDHP (S-binaphthyl diylhydrogen phosphate). Hindered rotation
about the bond connecting the naphthyl rings means there are two
non-superimposable forms even though there is no stereogenic center
(atropisomerism). Our earlier work supports
that the bile salts cholate and deoxycholate form anti-parallel dimers that
interact selectively with R- and S-BNDHP.
The NMR data localize BNDHP in the hydrophobic interior of cholate and
deoxycholate aggregates and show that the R,S-BNDHP enantiomers interact with
different outer edge surfaces of the bile aggregates. Recently, we turned to
NMR diffusometry as a hydrodynamic technique to observe how the binding of
R,S-BNDHP by bile micelles may affect the size of those micelles. Independent of chemical shifts and
NOE's, the translational diffusion provides helpful new information in these
systems. Figure
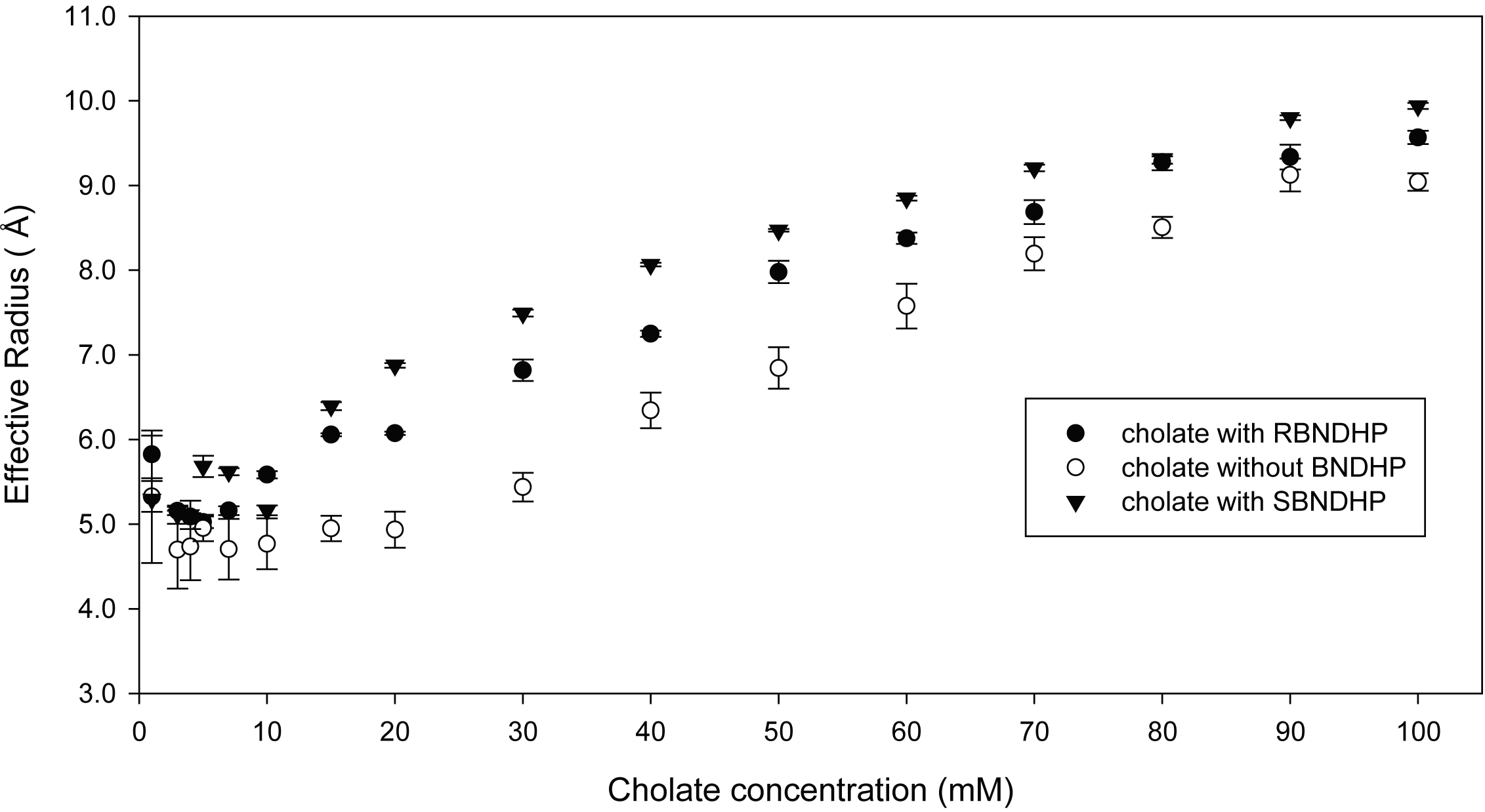
2. Plot of effective
radii of cholate micelles with (closed symbols) and without (open symbols)
BNDHP guest molecules. Effective
radii of cholate are calculated from NMR derived diffusion constants in
combination with measured viscosities. Diffusion data were
obtained over a wide concentration range of 0 to 100 mM cholate in the presence
and absence of the R,S-BNDHP analytes.
When interpreted via Stokes-Einstein analysis, one obtains effective
radii which represent a weighted average of all aggregation states of the bile
salt. (Figure 2): -
The greatest
difference in the bile salt radii occurs at ca. 20 mM cholate, a region we have
previously found maximizes the presence of primary micelles. -
The presence
of S-BNDHP results in higher average aggregate sizes, consistent with micellar
capillary electrophoresis experiments which show that the S enantiomer is
retained longer than the R. -
The radii of
the bile aggregates in the presence of either R or S-BNDHP (filled symbols) converge to the radii
observed in the absence of any analyte (open symbols), showing that high
concentrations of cholate begin to abolish the interaction with BNDHP. -
Cholate
undergoes a rapid increase in average size at about 30-40 mM, consistent with
the onset of secondary aggregation. In sum, we see direct
hydrodynamic evidence for primary and secondary aggregation in these data, and
are especially pleased to see that the effective aggregate size correlates
directly to chirally selective solubilization. We have also acquired
diffusometry data for deoxycholate
where similar correspondences were observed. We look forward to continued work in the application of NMR
diffusometry to the study of bile aggregates.
Copyright © American Chemical Society