AmericanChemicalSociety.com
Reports: GB3 47420-GB3: Bio-Inspired Two-Site Bimetallic Hydrocarbon Activation Catalysts
Stephen Contakes, Westmont College
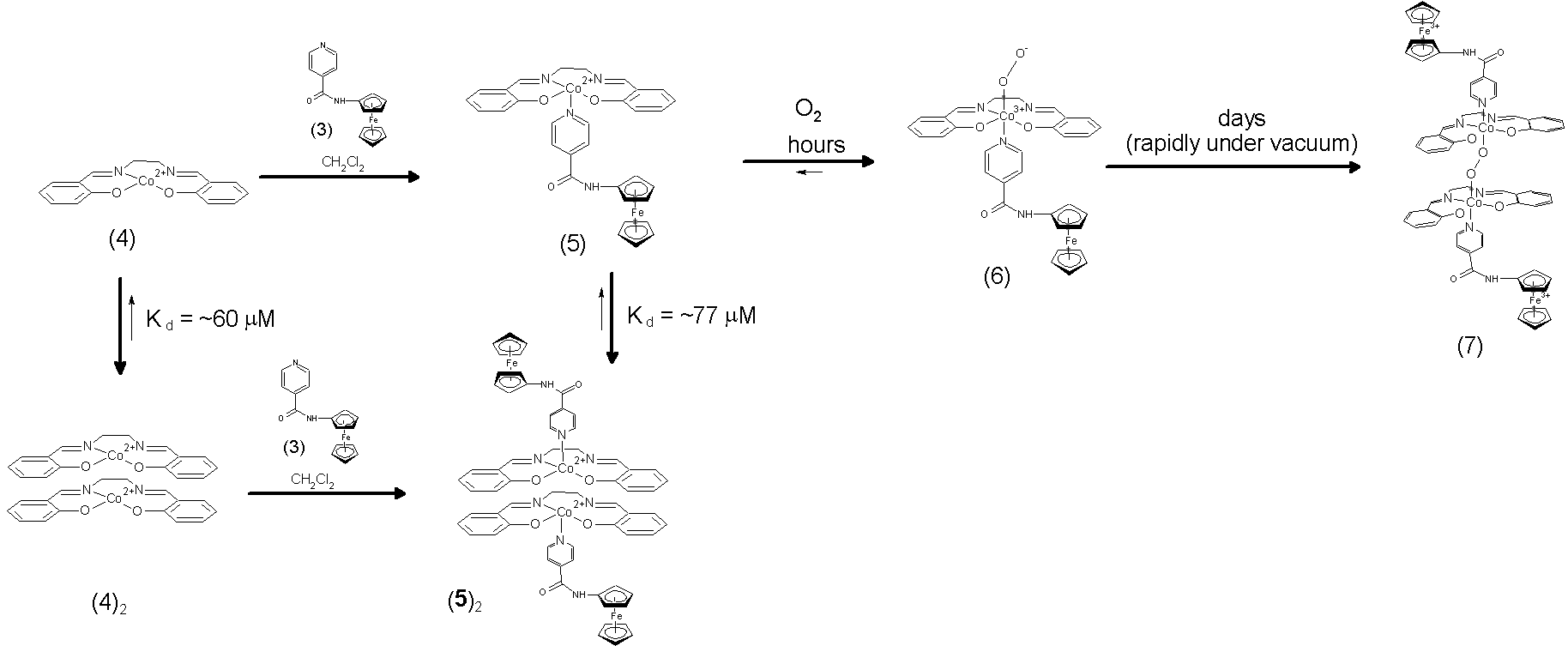
Research Progress. This overall goal of this research is the preparation of bimetallic oxidation catalysts that mimic enzymes by employing tethered redox “cofactors” to accomplish multi-electron oxidations. The original proposal called for the preparation of redox-active metallocene and Ruthenium-diimine derivatives of metal salen and porphyrin complexes and the characterization of their reactivity towards molecular oxygen. In our last report, we described the synthesis of a ferrocene-containing pyridine derivative, 3, and preliminary studies of its ability to function as an axial ligand in conventional salen complexes.
During
the present grant period, we prepared an adduct between 3 with Co(salen), 5, and
studied its reactivity towards molecular oxygen. Our results are summarized in the scheme
below: UV-vis
and IR studies of the reactivity of 5
towards molecular oxygen support the initial formation of a terminal dioxygen
complex (6) followed by its eventual
conversion to the bridging peroxide dimer (7). Analogous reaction sequences are well-established
reaction of Co(salen) derivatives with O2 in pyridine. The chief novelty of our system is that we
can control the stability of 6 by
manipulating 5's monomer-dimer
equilibrium. At low concentrations an
appreciable quantity of 5 is present
and both the formation of 6 and its
conversion to 7 in occurs over a
period of a few hours. At high concentrations
of 5, 6 is stable for several days.
We currently suspect that 6
is a CoII-superoxide complex based on its UV-vis spectrum and its
observed conversion to 7 in the
presence of 5. However, IR studies on air-exposed
dichloromethane solutions of 5 show
new bands at 1060 and 1027 cm-1 consistent with a terminal
superoxide as well as bands at 882, 818, and 792 cm-1, which may
indicate a weakened O-O bond. We intend
to resolve the issue of the O-O bond order (and the Fc oxidation state) during
the next grant period using a combination of 18O labeling
experiments, magnetic moment determinations, crystallography, and
spectroelectrochemistry. In
addition to the above efforts, we pursued two parallel strategies for
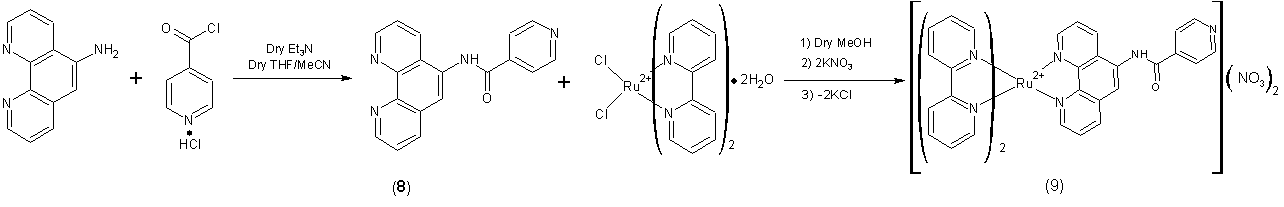
attachment of photoactive Ru-diimine complexes to Co(salen) complexes. Our first strategy involved that preparation
of 9, a [Ru(bpy)2(phen)]2+-analogue
of 3, by reaction of isonicotinoyl
chloride with [Ru(bpy)2(5-aminophen)]2+, as shown below. The
photochemical and redox properties of 9
were studied using UV-vis spectroscopy, emission spectroscopy, transient
emission spectroscopy, and cyclic voltammetry and found to be comparable to
other Ru-diimine complexes. Unfortunately,
we have so far been unable to study complex formation between 9 and Co(salen) due because the low
overall yield (~3%) has prevented us from obtaining enough to study its ability
to form adducts with M(salen) complexes and metalloporphyrins. During the next grant period, we hope to
obtain the desired quantities through a repeat of our current synthesis as well
as increase the overall yield by exploring alternate conditions. Our
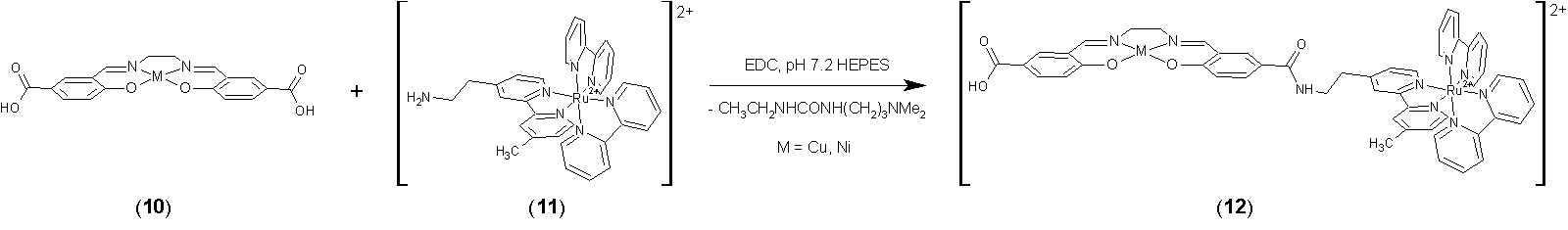
second strategy for the conjugation of photoactive Ru-diimine groups into
M(salen) and related complexes involves EDC coupling between amine derivatives
of [Ru(bpy)2(phen)]2+ or [Ru(bpy)3]2+ and carboxylic acid derivatives of metal
salen complexes, as shown schematically below. The
synthesis of Co, Cu, and Ni variants of 10
was accomplished during the present grant period. Initial attempts to couple [Ru(bpy)2(5-aminophenanthroline)](NO3)2
to carboxylic acids gave low yields, presumably due to deactivation of the
amine by the phen and Ru2+ center.
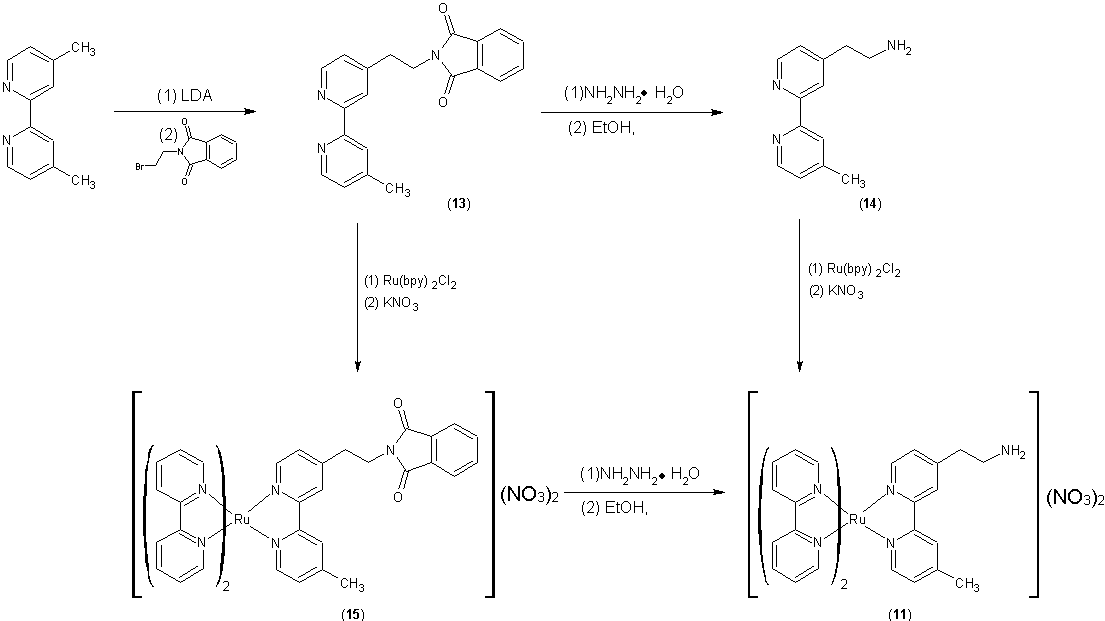
Consequently, we synthesized 11,
an aliphatic amine derivatives of Ru(bpy)2(4,4'-Me2bpy)](NO3)2,
using the synthesis shown below: Our
initial efforts focused on the synthesis of 11 from 4,4'-Me2bpy via 13 and 14. However, the yield was extremely low due to
the poor yield for formation of 14
and significant deamination of 14 to
give 4-ethenyl,5-Mebpy during the preparation of 11. Because we surmise that
deamination is occurring when the primary amine group coordinates to Ru, we are
currently pursuing an alternate synthesis involving the metallation of 13 followed by deptrotection of the
amine to give 11. Career impact. Since In addition to my own
professional development, the grant provided three different undergraduate
students with research experience during the summer of 2010, all of whom are
continuing work on the project this year.
These students, Bryan Brautigham, Lauren Bernau, and Ashley Greenawalt,
will be completing their studies this year and are currently applying to
graduate, medical, and dental schools, respectively. In addition, Lauren is planning to present a
paper on this work at the 2010 Southern California Conference for Undergraduate
Research. I anticipate using the remaining
grant funds to purchase 18O2 and for the support of 1 or
2 research students during the summer of 2011.
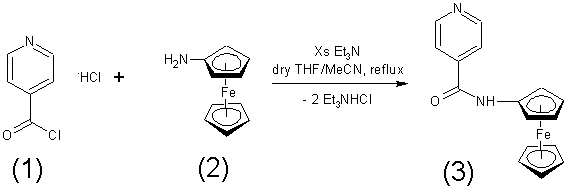
Copyright © American Chemical Society