AmericanChemicalSociety.com
Reports: DNI3 49296-DNI3: Structural and Functional Modeling of Nickel Superoxide Dismutase
Michael Jensen, Ph.D., Ohio University
1. Introduction. We prepared a series of hydrotris(3-phenyl,5-methylpyrazol-1-yl)borato (i.e., TpPh,Me) nickel(II) complexes with anionic chelating dithioacid donors [e.g., O,O′-dialkyldithiophosphates (RO)2PS2-, organoxanthates, ROCS2-, dithiocarbamates, R2NCS2- and dithiolene, 1,2-C6H4S22-] as models for the (N2+1S2)3- square-planar/pyramidal ligand field in nickel-dependent superoxide dismutase. As detailed in our proposal, dithiocarbamates yielded particularly robust Ni(II) complexes exhibiting a directly observable axial base equilibrium with concomitant spin crossover, as well as quasi-reversible one-electron redox couples at modestly anodic potentials. We have structurally characterized several allogonic spin isomers, and also prepared a rigorously square-planar analog by CF3COOH acidolysis of the axial pyrazole arm. These results enabled us to directly probe the effects of geometry and spin on the redox properties of the model system. An unexpected result was the observation of cooperative, non-allogonic spin crossover for Ni(II) in the solid state, which will receive further attention.
2. Impact. The results we obtained were crucial in support of a pending application for a multi-year grant from the National Science Foundation. One graduate student, Mr. Huaibo Ma, was supported full-time in his fourth year of graduate study, leading to greatly enhanced productivity in his dissertation research. Two other students contributed to the project in a part-time role. Two manuscripts are under preparation that will detail the results we obtained in the initial year of PRF support. Finally, the travel funds enabled my research group to attend the American Chemical Society National Meeting in Washington, DC in August 2009, where we presented one oral and two poster presentations. Hence, the PRF support has had a dramatic impact on our research productivity, future funding prospects, and dissemination of results. We expect these beneficial effects will continue in the second year of funding.
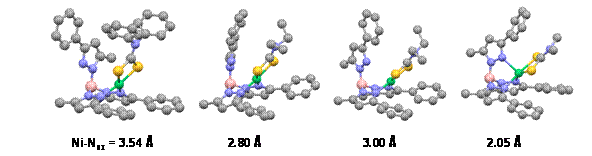
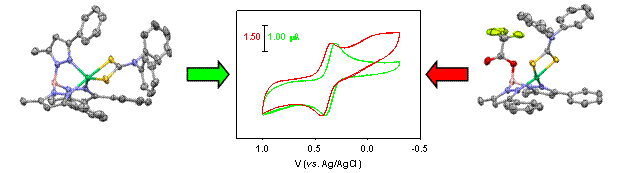
3. Research progress. Huaibo prepared a number of TpPh,MeNiS2CNR2 complexes that are primarily pentacoordinate and paramagnetic in solution, but equilibrate quite readily (DGo ≈ 0.5 kcal/mole) with a square-planar diamagnetic geometry with a detached axial pyrazole arm. As shown in the figure below, this flat energy surface allowed structural characterization of several spin-isomeric allogons in the initial funding year. He had previously demonstrated both thermal and proton-dependent equilibration of the limiting four- and five-coordinate geometries, but more recently also isolated the diamagnetic acidolysis product [{(F3CC(O)OB(H)(3-Ph,5-Me-pz)2}NiS2CNPh2], which allowed comparative cyclic voltammetry of dithiocarbamate complexes with and without an axial base. These data indicate that removal of the axial pyrazole only slightly
perturbs initial oxidation, but strongly affects the reversibililty, indicating loss of stability in the oxidized product. This fundamental result is the basis for one manuscript under preparation. As proposed, Huaibo also prepared complexes of respectively less and more basic dithiophosphate and dithiolene ligands, which adopt limiting paramagetic and diamagnetic geometries, respectively. Structural characterizations have been thwarted by limited stability of these products to hydrolysis and oxidation, respectively.
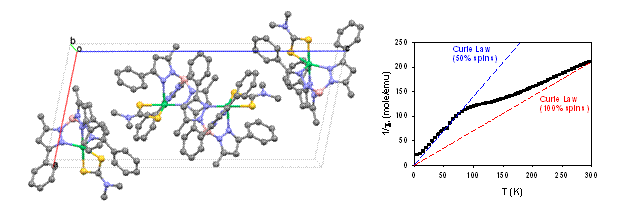
Given the propensity of the dithiocarbamate complexes to undergo allogonic spin crossover in solution, we also examined their solid state behaviors. In the case of TpPh,MeNiS2CNMe2, we were delighted to find clear evidence of cooperative, non-allogonic spin crossover. The unit cell contained independent five-coordinate molecules that exhibited intermolecular p-stacking and/or short van der Waals contacts. One of these exhibits a significant axial Ni-N bond elongation in a 123K structure (2.40 Å) relative to room temperature data (2.15 Å), with corresponding bond contraction in the equatorial N2S2 plane, which is oriented parallel to a uniquely long unit cell axis. Magnetization data obtained in collaboration with Prof. Gordon Yee of Virginia Tech confirmed equilibration of half the spins with a critical temperature near 120 K, and also indicated crossover of the second molecule at lower temperatures. This unprecedented result is the basis for a second manuscript, and is also a new research opportunity for our group. Spin crossover of octahedral d4-d7 complexes is being intensively investigated as a means to obtain molecular switches for nanoscale computing, data storage and displays. Our robust tetragonal d8 Ni(II) complexes afford comparably large ligand field distortions, albeit axial instead of isotropic, which together with the smaller change in electron configuration should afford more favorable crossover entropy and therefore access to higher critical temperatures.
4. Future directions. With the remaining support, we will expand our investigation to include dithiocarbamate ligands with polar substituents. This will induce aqueous solubility in model complexes, facilitating a search for active SOD catalysts in standard assays. We will attempt to isolate and characterize oxidized, formally Ni(III) species germane to such catalysis and examine their electronic structures. Moreover, we will examine prospects for using the Ni(II) complexes as spin crossover materials.
Copyright © American Chemical Society