AmericanChemicalSociety.com
Reports: G1 47698-G1: Binuclear Metal Complexes as Asymmetric Catalysts
Daniel Seidel, Rutgers, the State University of New Jersey
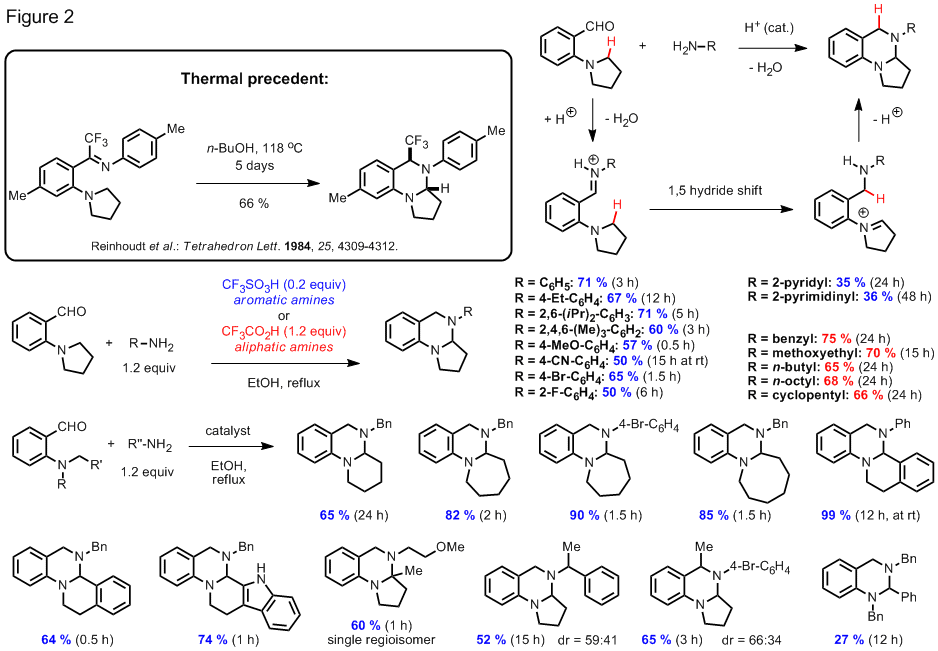
The activation of relatively unreactive C–H bonds for C–C or C–X bond formation frequently relies on the intervention of organometallic intermediates and often involves oxidative processes. In the course of pursuing our originally proposed research, we have discovered a new alpha-functionalization reaction of cyclic amines that proceeds without the involvement of transition metals or other additives (Figure 1). In this redox-neutral condensation reaction, a comparatively unreactive C–H bond alpha to a ring-nitrogen is replaced by a C–N bond, concomitant with the formation of a new ring system. This thermally promoted reaction between an aminoaldehyde and a cyclic amine results in the formation of a ring fused aminal and thus provides convenient access to this structural motif. The reaction conditions resemble those used in Friedlaender condensations of aminoaldehydes and ketones to form quinolines in which pyrrolidine is frequently used as a base promoter. Some of these aminals represent reduced versions of quinazolinone alkaloids, compounds that have attracted significant attention in the synthetic community due to their diverse array of biological activities. Selective oxidation of ring fused aminals provides rapid access to this structural motif which allowed for the synthesis of the natural products deoxyvasicinone and rutaecarpine in just one additional step.
We have succeeded in preparing
other aminal products that represent regioisomers of the compounds shown in Figure
1. In an overall redox neutral process
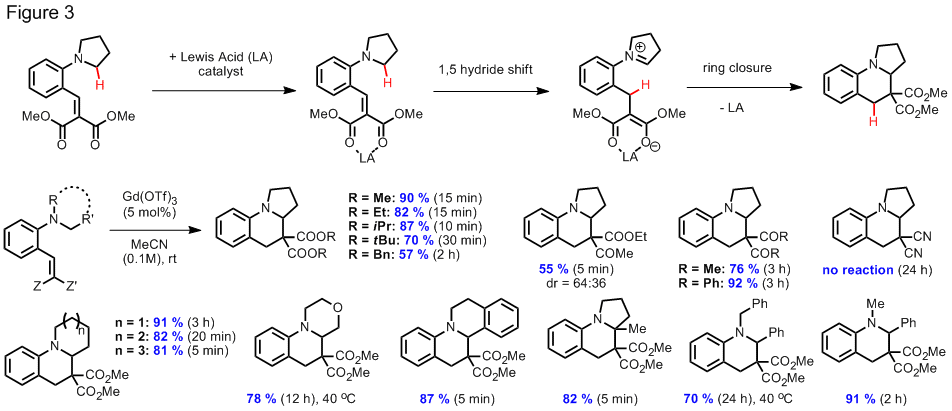
that is believed to involve a 1,5-hydride shift event,
aminobenzaldehydes are condensed with aliphatic or aromatic amines in an acid promoted
process. The scope of this reaction is
summarized in Figure 2. Reactions of
aminobenzaldehydes with aromatic amines were found to proceed well in the
presence of catalytic amounts of triflic acid whereas the corresponding
reactions with aliphatic amines worked best when an excess of trifluoroacetic acid was employed.
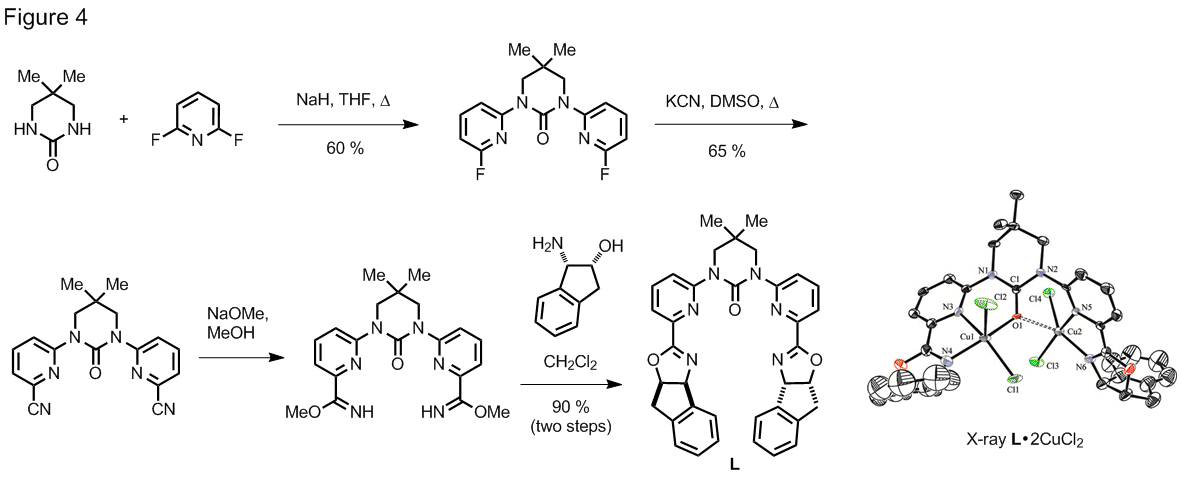
In a related study, we have found
that catalytic amounts of various Lewis acids facilitate rearrangement of alkylidene malonates to complex tetrahydroquinolines at
room temperature (Figure 3). Scandium
triflate readily catalyzed this transformation.
Albeit less efficiently, several main group and transition metals were also
found to catalyze this rearrangement.
Remarkably, gadolinium triflate showed striking
rate acceleration as compared to scandium triflate (up to 88 fold rate
acceleration under optimized conditions).
Again, a 1,5-hydride shift is thought to be involved
in this redox neutral reaction.
Tetrahydroquinolines are at the core of many biologically active
materials and the compounds prepared in our study would be difficult to obtain
by other means.
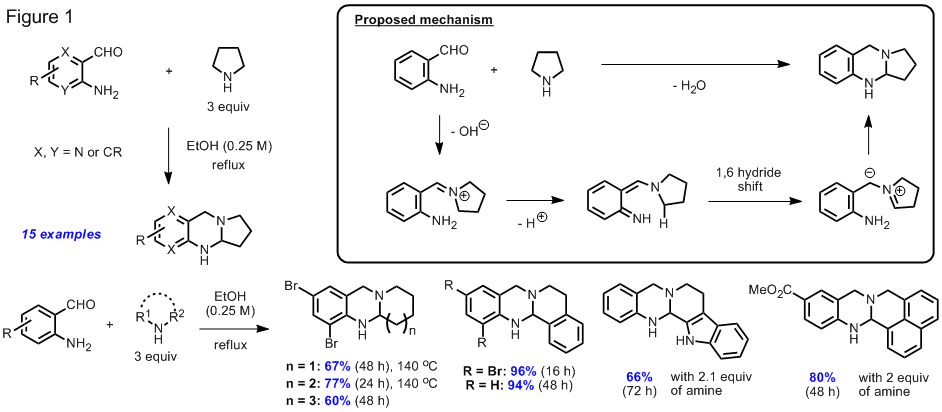
The goal of our originally
proposed research was the synthesis of new ligands
capable of stabilizing bimetallic complexes for potential use in asymmetric catalysis. This project was based on the assumption that
the activation of a single functional group by simultaneous interaction with
two metal ions could offer distinct advantages over the use of a single metal
center. In the course of our studies, we
successfully prepared a number of bisoxazoline ligands, such as the ligand L shown
in Figure 4 . Preliminary
experiments have shown that the new ligand readily
forms complexes with various zinc, nickel and copper salts. The binuclear nature of these complexes was
confirmed by an X-ray crystal structure of a bis-copper
complex derived from L and two equivalents of copper(II)chloride.
This PRF grant has supported a
number of graduate students over the past two years. In addition, funding by the PRF enabled us to
obtain a sufficient amount of preliminary data on this project, ultimately resulting
in funding from the NSF.
Copyright © American Chemical Society