AmericanChemicalSociety.com
Reports: UNI3 49511-UNI3: Development of Light-Harvesting Metallopolymers with Tunable Optical and Electronic Properties
Manal A. Rawashdeh-Omary, PhD, Texas Woman's University
GENERAL IMPACTS:
This award has had strong impacts on the following:
· Graduation of two MS students and job placement for both.
· Two new MS students and three undergraduates joined the PI's group and started training.
· Most trainees on the project were women and minority with low-income background.
· Four manuscripts, one published, another submitted, and two in preparation that acknowledged PRF support.
· Four conference presentations and one invited seminar that acknowledged PRF support.
· Application of the PI for early tenure and promotion to Associate Professor.
· Three new collaborations with PhD-granting institutions (UT-Austin, UT-Arlington, and UNT).
TECHNICAL PROGRESS:
1. New classes of Pt(II)-based metallopolymers have been synthesized and characterized. These have included the two following sub-classes:
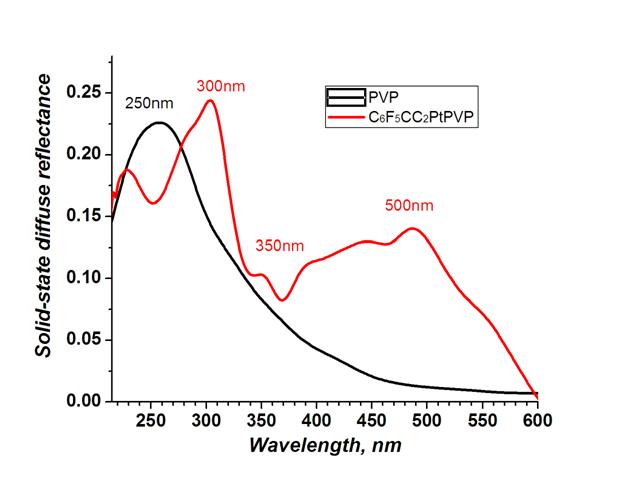
a. Polyvinylpyridine-based metallopolymers: PVP2Pt(CCPh)2; PVP2Pt(CCPhF5)2; PVP2PtCl2; PVP2PtQDT (where PVP = poly(4-vinyl)pyridine; QDT = quinoxaline-2,3-dithiolate). The resulting metallopolymers exhibit charge transfer absorption bands that are red-shifted from the p-p* absorption bands in the parent PVP polymer, as illustrated in Figure 1 for PVP2Pt(CCPhF5)2. The absorption of these Pt(II)-PVP metallopolymers cover the majority of the visible region, whereas PVP alone exhibits only UV absorption.
Figure 1. Solid state diffuse reflectance spectra of the metallopolymers complex (C6F5CC)2PtPVP2 (red) and polymer PVP (black).
b. Plythiophene-based metallopolymers: These were attempted by two different reactions (P3HT + PtCl42- or THT2PtCl2, where P3HT = poly(3-hexyl)thiophene and THT = tetrahydrothiophene). However, no evidence of a complete reaction was found in such attempts so no further derivatives were sought. This is possibly due to low basicity of P3HT, which although a better chromophore compared to PVP, is not as good a p-acceptor and its lone pairs are not as accessible.
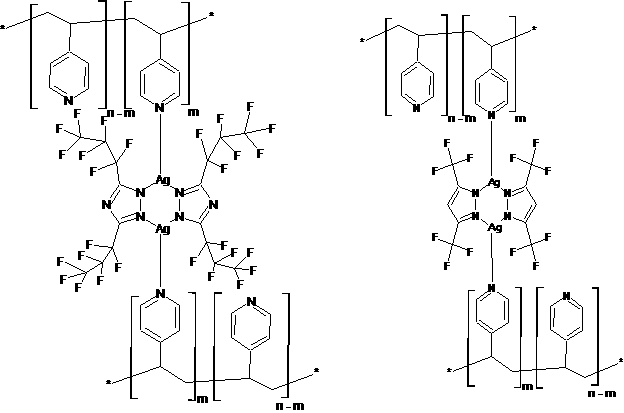
2. New classes of Cu(I)- and Ag(I)-based metallopolymers and precursors thereof have been synthesized and characterized. These metallopolymers were obtained by reacting metallomacrocyclic [MAz]3 azolate trimers with PVP, leading to PVP[MPz]2 products (where M = Cu or Ag; Az = Tz or Pz); see Figure 2. The optical properties of these metallopolymers were such that they exhibit near-UV absorption and visible photoluminescence so their application in solar energy is limited. Nevertheless, the success of the synthetic strategy to allow d10 metal centers, including cost-effective metals of silver and copper instead of platinum or gold, is promising for further modification of the ligand system to lead to visible light-harvesting analogues.
Figure 2. Structure of Ag(I)-based metallopolymers with triazolate (left) and pyrazolate (right) co-ligands. Analogous Cu(I)-based metallopolymers of this type have also been synthesized and studied.
3. New classes of Ru(II)-based metallopolymers designed and started synthesis for. The retro-synthesis scheme for an example of these metallopolymers is shown in the following scheme:
[Ru(bpy)(PVP)2(bdt)] <== [Ru(bpy)(py)2(bdt)] <== [Ru(bpy)(py)4]Cl2 <== [Ru(bpy)3]Cl2 (where bpy = 2,2'-bipyridine; bdt = o-benzenedithiolate)
While Ru(II) small molecules have received tremendous attention for solar cell applications, metalopolymer analogues remain lacking prior to this effort that we have just started. In addition to expanding the material regime via the coordinating polymer PVP, the presence of both p-acceptor and p-donor ligands in the target metallopolymers (bpy and bdt, respectively, in the above example) as well as the low symmetry are attractive features in these new materials.
4. New classes of Au(I)-based metallopolymers and precursors thereof have been characterized. These have included the two following sub-classes:
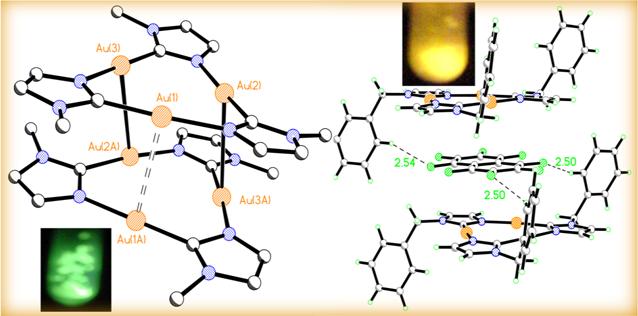
a. The p-basic trinuclear complex [Au(m-C2,N3-bzim)]3 (where bzim, 1-benzylimidazolate) and its charge transfer adduct with the p-acidic organic molecule octafluoronaphthalene. The aurophilic interactions in the trimer alone are replaced by cooperative p acid-based quadrupolar and H...F dipolar interaction, which lead to further red-shifting of its electronic spectral bands (Figure 3). This work has already been published while metallopolymers expansion using strategy #2 above is ongoing. We plan to attempt that strategy with not only PVP but also P3HT because of the known thiophilic nature of gold.
Figure 3. Crystal structure and luminescence of [Au(m-C2,N3-bzim)]3 (left; benzyl substituents are truncated for clarity) and its charge transfer adduct with octafluoronaphthalene (right).
b. Au(I) molecular and metallopolymers complexes with N-heterocyclic carbenes. These studies have been in collaboration with the Cowley group at UT-Austin, whose role is to synthesize the carbene complexes, whereas the PI's group has been studying the optical properties toward solar cell applications. So far we have characterized the absorption and emission properties of bithiophene-substituted imidazolyl carbene free ligands and Au(I) complexes, both of which were found to be superior chromophores, while ongoing work is targeting the corresponding metallopolymers obtainable by electropolymerization of the monomeric complexes.
Copyright © American Chemical Society