AmericanChemicalSociety.com
Reports: UR4 49399-UR4: Demystifying Microwave Assistance in Homogeneous Catalysis: Chemical Kinetics of Microwave-Heated Palladium Reactions
Andrew W. Holland, PhD, Idaho State University
Many palladium catalyzed reactions are reported to enjoy dramatic acceleration when conducted using microwave rather than conventional heating methods, even at comparable temperatures and under otherwise similar reaction conditions. The origin of this improvement is not understood in many systems, and the goal of this project is elucidate the origin of this effect by profiling reaction kinetics, rather than measuring overall yields, to distinguish differences in catalyst lifetime, induction periods, average rates, and other possible effects of microwave conditions.
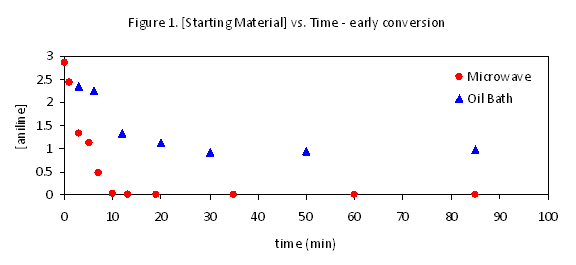
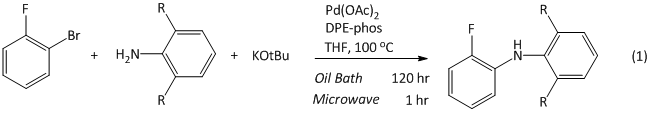
We have initiated this work by investigating a Buchwald-Hartwig reaction previously reported to proceed to completion in 30 minutes with microwave heating, and in 5 days with oil-bath heating (eq 1). Upon further examination we have found reaction progress, under both sets of reaction conditions to be irreproducible and strongly dependent on catalyst batch, but we have confirmed the existence of differences between microwave and conventionally heated reactions. Strikingly, the majority of conversion occurs at very early reaction times with both methods, with the vast majority of the reaction time corresponding to a much slower rate and only and only 10-40% of the total conversion observed (Figure 1). This suggests that the duration of this initial burst of reactivity, perhaps correlating to the lifetime of the most effective catalyst generated, might be the key difference between the two reaction conditions. We are simultaneously working to collect similar data on other subject reactions.
In the course of investigating these early reaction times more
carefully, we have worked to overcome challenges in collecting accurate kinetic
data for microwave reactions. The first reaction for investigation was chosen
for its moderate reaction time and temperature, which we hoped would allow convenient
alternation between heating and analysis, but shorter reaction times lead to
relatively more significant warming and cooling periods which frustrate efforts
at careful time and temperature control. As such we have validated an
alternative approach in which multiple simultaneous reactions are quenched at
different final reaction times, minimizing the warming and cooling cycles and
providing more meaningful time and temperature data.
Copyright © American Chemical Society